Welcome back to the “52 in 52” series. This collection of posts features recently published must-know articles. Today we look at the DAWN trial.

Author: Austin G. MacDonald, MD (Resident, Emergency Medicine Physician, San Antonio, TX); Brannon L Inman, MD (Critical Care Fellow, Orlando, FL) // Reviewed by: Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)
Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct
AKA: The “DAWN” Trial
Clinical question:
Does the addition of thrombectomy to standard medical care within 6-24 hours after an acute stroke result in better functional outcomes at 90 days compared to standard medical care alone?
Study design:
- Randomized, prospective, multicenter, open-label with a Bayesian adaptive-enrichment with blinded assessment of end points.
- A second primary outcome was added at the 30-month time frame by request of the FDA, the trial was still blinded at that time.
- The blinded assessment of the primary outcome was done through both in-person interviews and over the telephone.
- There was a 1:1 randomization, done by a randomization software, patients stratified by: age, mismatch criteria, time from onset, and site of occlusion. No crossover permissible after randomization.
- An intention to treat analysis was maintained.
- RAPID software was used to measure the infarct and ischemic size on perfusion/diffusion images.
- POWER: 86% to detect a 1-point difference in the group’s first primary outcome.
- The trial was stopped after the first interim analysis, with no change in the criteria for inclusions/exclusion.
- Limited the subject size to 500, with no more than 20 sites allowed outside the U.S.
PICO:
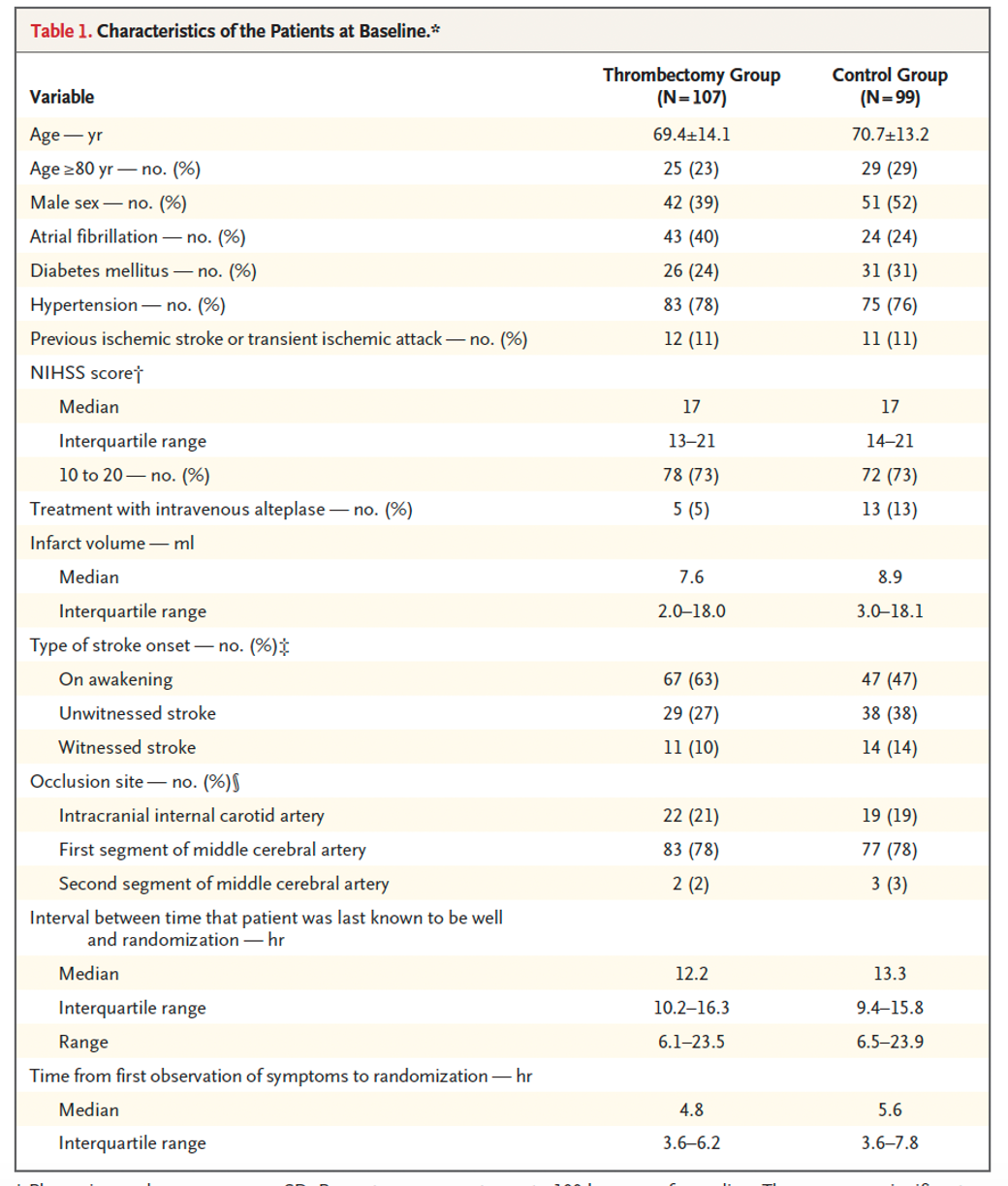
Population: 206 patients were enrolled with 107 in the intervention group and 99 in the control.
– Inclusion criteria:
- Included patients aged 18 or above, who had a baseline NIHSS 10 or greater (obtained within one hour before measuring infarct volume) across 26 hospitals in the United States, Canada, Europe, and Australia.
- Patient had to be able to be randomized between 6-24 hours after last known well.
- Pre-stroke disability had to be insignificant measured by mRS (modified Rankin Scale) of 0 or 1.
- No evidence of intracranial hemorrhage on CT or MRI.
- No evidence of an infarct involving more than one third of the territory of the MCA on CT or MRI at baseline.
- Life expectancy had to be 6 months or greater.
- Presentation consistent with diagnosis of an acute ischemic stroke, with the patient meeting criteria of:
- Failing IV t-PA therapy; OR
- Patient is contraindicated for IV t-PA administration.
– Exclusion criteria:
- Medical history of severe head injury with lasting neurologic deficit in past 90 days.
- Prompt correction of NIHSS to less than 10 or vessel recanalization before randomization preformed.
- Pre-existing neurologic or psychiatric disease that would act to confound evaluation pre and post intervention. The example they give is taking Aricept in the setting of known dementia.
- Imaging revealing ICH, infarct greater than one third MCA, cerebral vasculitis, tumor, multiple vascular occlusion, pre-existing stent of treatment intervention, flow limit carotid dissection.
- Laboratory values consisted of blood glucose less than 50 or greater than 400, hemoglobin less than 7, platelets less than 50,000, sodium less than 130, potassium less than 3 or greater than 6, creatinine greater than 3 (unless on previous dialysis), and INR greater than 3 or PTT more than 3 times normal.
- Excluded if received factor Xa inhibitor in past 24-48hrs unless they had a normal aPTT.
Intervention:
- Thrombectomy with Trevo (a specific brand of thrombectomy device) + medical management (more detail below)
- 2,000-unit bolus followed by 500u/hr of Heparin used during the procedure
- *No use of other IV and IA thrombolytics or antiplatelets (other than aspirin) until after follow up imaging completed.
Comparator:
- Medical management only with IV heparin prohibited until after the 24hr imaging is performed.
- Medical management permitted for both groups:
- No IA therapies, otherwise, medications at discretion of treating physician and standard of care.
- Aspirin is the only anti-platelet allowed in the first 24hrs after randomization.
- Patients previously treated with antiplatelet agents or combination antiplatelet therapy, may continue after post procedure imaging is completed if in the provider’s opinion the benefits of continued therapy outweigh the risks of potential neurological deterioration.
- Subcutaneous Low Molecular Weight heparin is permitted for DVT prophylaxis per the facilities standard of care.
Outcome:
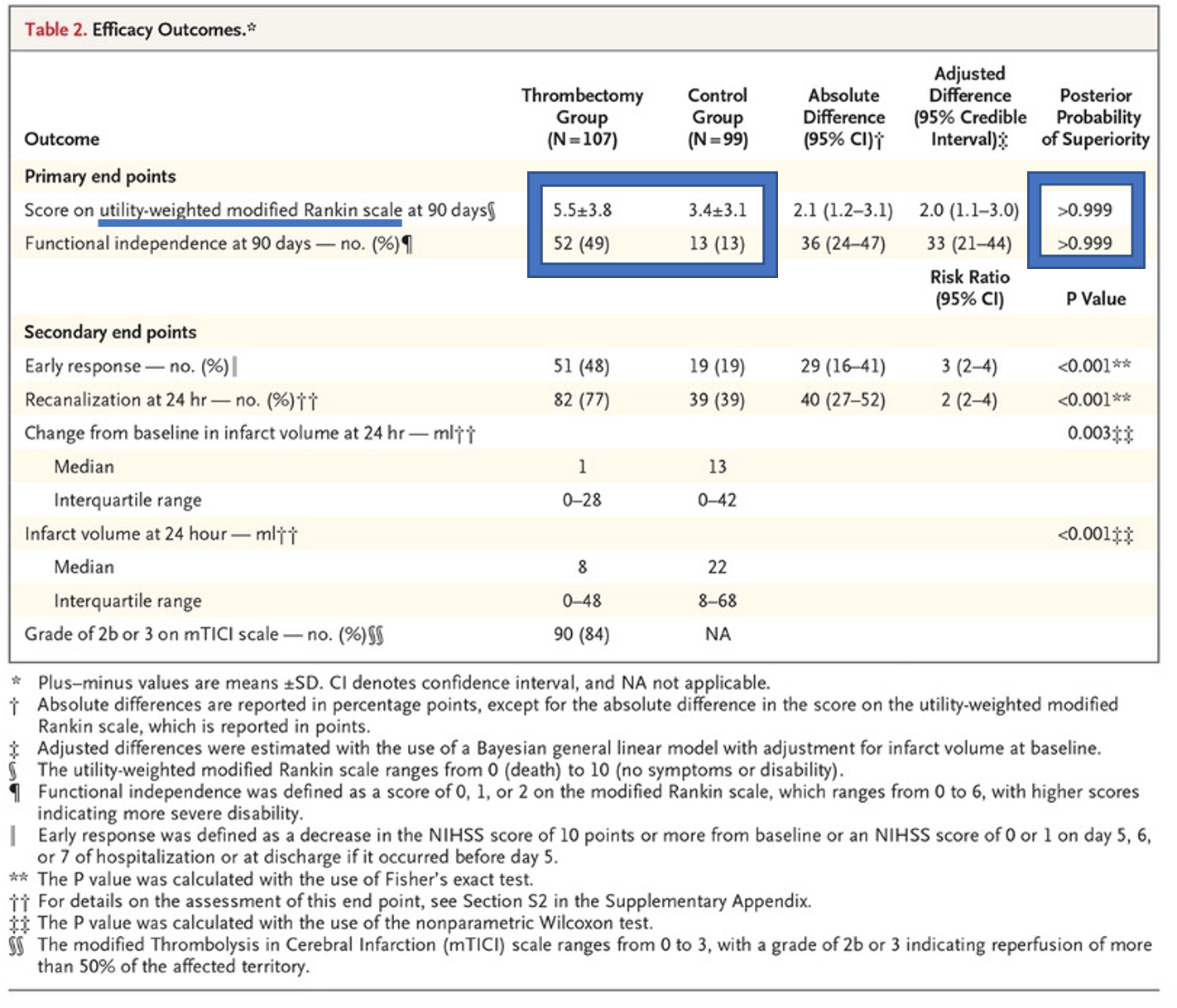
- Statistically significant difference in co-primary outcomes (weighted modified Rankin Scale at 90 days and good functional outcome at 90 days defined by mRS 0-2)
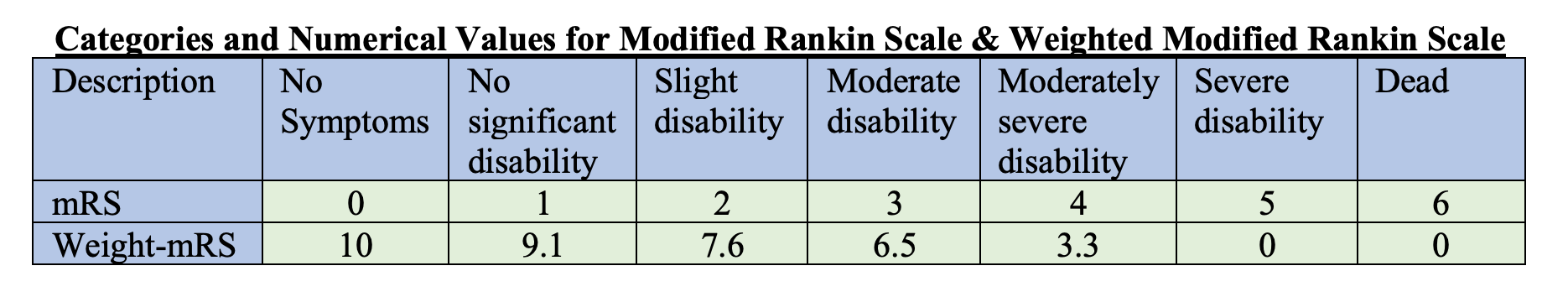
- Note that the weight modified Rankin Scale was used for the first primary outcome, aka the higher the number the better the outcome on the scale.
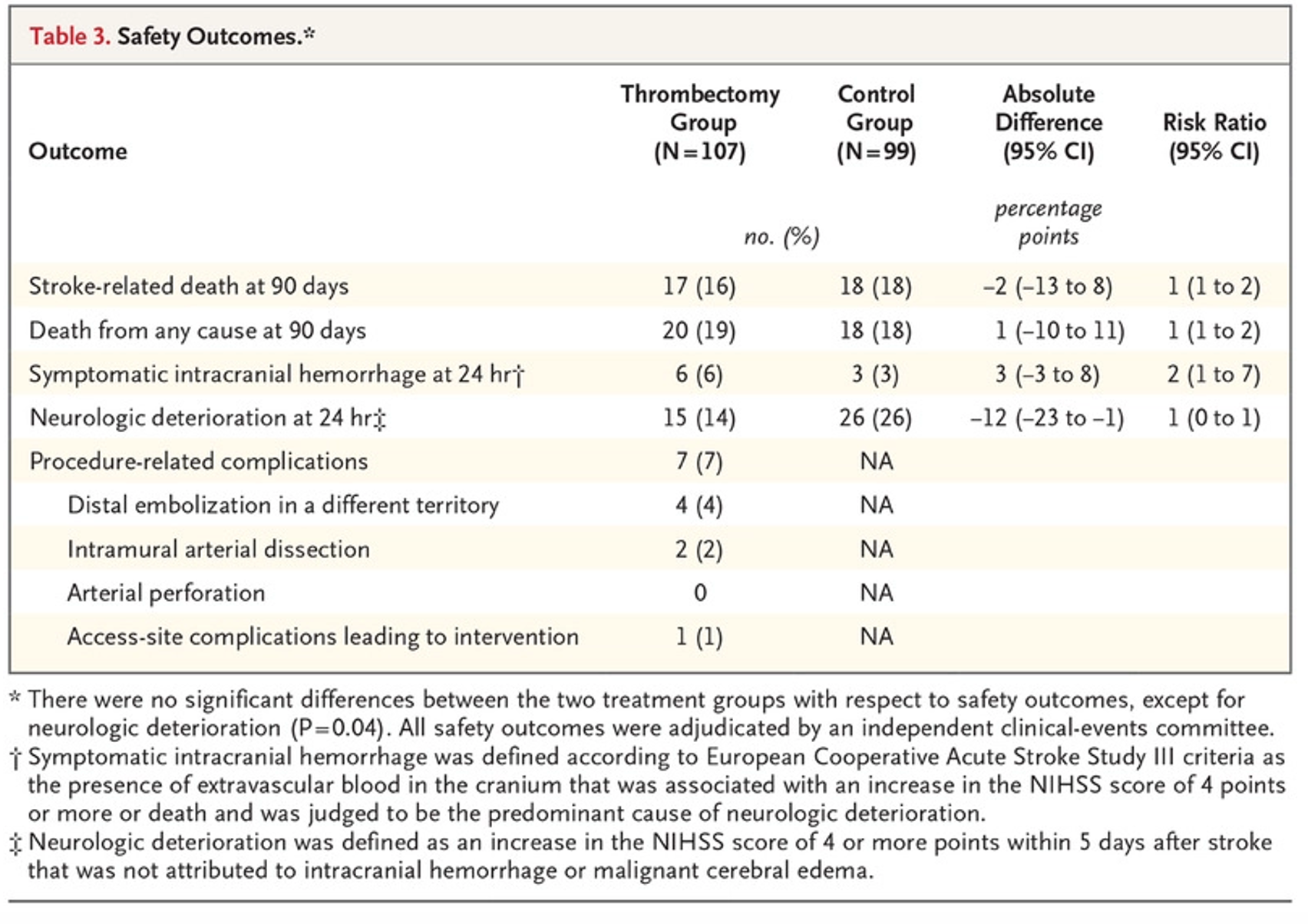
- No statistical significance in primary patient safety outcome (stroke-related mortality at 90 days)
Randomization:
- The groups were relatively equal, other than higher rates of atrial fibrillation and stroke at the onset of wakening in the experimental group (43 to 24 and 67 to 47, respectively). The control group had a higher rate of t-PA treatment (5-13).
Take away:
- Positive trial
- The study used superior imaging techniques and extensive stroke care that may not be readily available at smaller facilities. The trial also only looked at one type of thrombectomy device, the Trevo.
- It should be noted that this study was industry funded:
- It was sponsored by Stryker Neurovascular, who supplied the study funding, devices for thrombectomy, as well as data analysis. They did have supervision from independent statisticians.
- There were no statistically significant differences in safety outcomes, but it should be noted that symptomatic ICH at 24hrs was 3% higher in the experimental group, and death from all cause at 90 days was 19% and 18% in the experimental and control groups respectively.
- Primary outcomes were patient-centered outcomes. At the request of the FDA, Functional independence originally a secondary outcome was made into a co-primary outcome. It matters to patients that 49% of individuals in the experimental group had functional independence at 90 days compared to only 13% in the control group.
- More data using various thrombectomy devices paired with a more generalizable collection of clinical stroke presentations are needed to validate this study.
My take:
In patients presenting with ≤ 24 hours of stroke symptoms with a high NIHSS even with a small infarct volume I will pursue thrombectomy evaluation. I will continue to obtain neuroimaging on all patients with clinical signs and symptoms of ischemic stroke.
Reference:
- Nogueira RG, et al., DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 378, 11–21 (2018). DOI:10.1056/NEjMoa1706442