Author: Adam Roussas, MD, MBA, MSE (EM Resident Physician, Cook County Health) // Reviewed by: Jamie Santistevan, MD (@jamie_rae_EMdoc, EM Physician, Presbyterian Hospital, Albuquerque, NM); Manpreet Singh, MD (@MPrizzleER); Brit Long, MD (@long_brit)
Case
A 40-year-old female presents to the emergency department for palpitations and lightheadedness. She has a history of depression on citalopram, migraines on amitriptyline, and was recently prescribed tramadol after she broke her wrist. She is well appearing, and while being placed on the monitor becomes anxious stating the symptoms are recurring. An ECG is performed and is shown below:
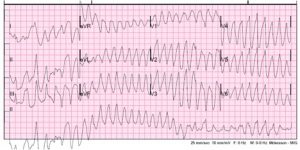
Figure 1. Adapted from Dr. Smith’s EKG Blog. Source: http://hqmededecg.blogspot.com/2013/10/polymorphic-ventricular-tachycardia.html
Her blood pressure is 120/80 mmHg and her heart rate is 170 bpm. The patient is awake and protecting her airway. She denies chest pain. You identify the rhythm as polymorphic ventricular tachycardia (PVT). Defibrillator pads are placed and you give 2 g IV magnesium over ten minutes. The patient remains stable but does not respond. You give another 2 g of magnesium IV with termination of the rhythm. Another ECG is obtained and shown below.

Figure 2. Adapted from wikipedia. Source: https://en.wikipedia.org/wiki/Long_QT_syndrome
As you are calling the ICU and cardiology team, the patient has recurrence of her symptoms and repeat ECG shows return of the PVT. She is still hemodynamically stable. What do you do?
Initial Treatment and Prevention of Recurrence
Background
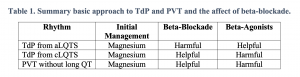
The initial ECG above shows polymorphic ventricular tachycardia (PVT). Although commonly referred to as torsades de pointes (TdP), not all PVT is TdP. A diagnosis of TdP is made by the combination of polymorphic ventricular tachycardia in addition to a prolonged QT-interval.This is an important distinction to make. Any PVT will likely be presumed to be TdP and treated as such initially without causing any harm; however, treatments for refractory PVT require a reliable measurement of the QT interval. Treatment of PVT without prolonged QT will involve QT-prolonging agents such as amiodarone, procainamide, and beta-blockers (typical treatment for ventricular tachycardia). However, these medications must be avoided in patients with TdP, since the underlying cause of the rhythm is long QT. Similarly, the medications used to treat refractory TdP from acquired LQTS (aLQTS) will be harmful in treating PVT without long QT or TdP from congenital long-QT syndrome (cLQTS)1,2. Beta blockade, in particular, is a mainstay of treatment but has the potential to cause significant harm if initiated hastily.
Pathophysiology
Collecting as much history as possible prior to initiating further treatments for recurrent or refractory TdP could be the difference between a good save and a clean kill. This is due to differences in how TdP is triggered in aLQTS versus cLQTS. In both instances, TdP occurs when an early after-depolarization (EAD) stimulus is generated during the repolarization phase of the cardiac action potential (also known as R on T phenomenon). The resulting electrical stimulus produced remains greater than the action potential threshold for multiple beats and appears on a 12 lead EKG as a malignant ventricular arrhythmia. This sequence is demonstrated in figure X below.
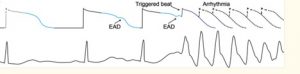
Figure 3. Adapted from Wilde et al. Illustration of EADs occurring during repolarization (R on T). The above strip demonstrates a cardiac action potential and the blow the rhythm strip of single lead EKG. At the “trigger beat,” which occurs at an elevated resting potential, the myocardial action potential is never able to return to a state below the action potential threshold and thus the development of malignant arrhythmia.
In aLQTS, an unstable cardiac membrane is prone to EADs. When the QT interval is lengthened, these asynchronous depolarizations are more likely to occur during repolarization and evolve into ventricular fibrillation or PVT. Intuitively, agents that slow down the heart (beta-blockers) will lengthen the QT interval and increase the risk of developing a malignant arrhythmia. Contrast this with cLQTS: the QT interval is always prolonged and EADs are not just produced by an unstable myocardium, but also due to the patient’s underlying membrane channel malfunctions rendering their myocardium hypersensitive to sympathetic input. As such, treatment should be focused on inhibiting sympathetic flow (beta-blockade), which results in improved control due to limiting sympathetic inflow and reducing EADs. However, nodal blocking agents used to treat ventricular tachycardia without QT prolongation such as amiodarone or procainamide must, again, be avoided, as these are QT prolonging agents. A complete approach to treating cLQTS can be found in Wilde et al2. Our post will focus on the treatment of recurrent and refractory TdP in both medically stable and unstable patients with aLQTS.
1. Treat the Ventricular Tachycardia
Ultimately, TdP is ventricular tachycardia. Electricity can always be used as first-line treatment. Assess your patient and if there is any sign of instability, use electricity. It is recommended to use an unsynchronized setting as the defibrillator may not be able to track the R-wave in the polymorphic waveform. A 200J shock is reasonable – though a higher dose may be needed for larger patients. Hedging on the side of higher energy is appropriate, as an unsuccessful cardioversion may lead to ventricular fibrillation. Any pulseless patient should be treated per ACLS protocols.
2. GFR-driven magnesium bolus and drip protocol
Initial management protocols for TdP are built to prevent recurrence. A simplified standard protocol for ED management is below3.
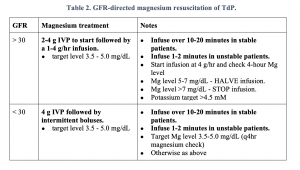
Protocols for stable patients typically recommend an initial 2 g IV push over ten minutes followed by an additional dose as needed and a 1-4 g/hr magnesium infusion. It is important to emphasize with your team that if pushed too quickly, rapid infusion of magnesium can result in hypotension. If the patient is unstable, the boluses may be given again as a slow push but over 1-2 minutes in addition to electricity. If no response at all, then begin to consider other diagnoses (torsades mimics include severe hyperkalemia, coarse ventricular fibrillation, atrial fibrillation with Wolff-Parkinson-White, or Takotsubo cardiomyopathy). If the response is appropriate, get another EKG and assess the QT interval. If the QT interval is prolonged, the patient is at high risk for recurrence – this is the purpose of the magnesium drip.
The same principles can be applied to pediatric patients with modified doses. Use 25-50 mg/kg (max 2 g) boluses over 60 seconds repeated every 5 – 15 minutes followed by a 0.5-1.0 mg/kg/hour infusion1.
Importantly, magnesium is not meant to affect heart rate or the QT interval, but is thought to stabilize the cardiac membrane via blockade of membrane L-type calcium channels resulting in activation of the Na+-K+ ATPase, subsequent reduction in activity of the Na+-Ca2+ exchanger, and activation of magnesium dependent Ca2+ ATPase4.
3. Treat the cause of acquired prolonged QT
Other causes of long QT should also be identified and treated. Common causes include hypokalemia, hypocalcemia, hypomagnesemia, and hypothermia3. Identify and discontinue all QT-prolonging medications. A list of common medications can be found below.

4. Optimize the potassium
The AHA suggests maintaining a potassium level of 4.5-5.0 may be beneficial, though this is supported by limited evidence.
Treatment of “Torsade Storm”
Torsade storm is defined as 3 or more episodes of TdP in 24 hours.
1. Ensure adequate magnesium repletion
If no infusion was given, a therapeutic level of magnesium was likely not obtained or not sustained. Magnesium is readily excreted by healthy kidneys and levels have been found to decrease significantly after only a few hours2. In relapsed patients on or off a drip, check a magnesium level. If below 3.5 mg/dL, the magnesium protocol above can be repeated.
2. Isoproterenol decreases the QT-interval
Medical overdrive pacing is speeding up the heart with chronotropic medications. The target heart rate is between 90-110 bpm, though rates as high as 140 bpm are sometimes required1. Pacing has been noted to be particularly useful in suppressing frequent self-limited episodes of TdP4. Although some sources state patients who are tachycardic or actively in TdP may not benefit, the case studies reviewed almost exclusively used isoproterenol in refractory cases with elevated heart rates or to break TdP1,3,4. The table below provides typical doses of medications that can be used to chemically pace the heart in acquired TdP.

Isoproterenol should be used with caution. It is a non-selective beta-1/beta-2 agonist and is contraindicated in patients with cLQTS or PVT without prolonged QT. It is further contraindicated in patients with acute myocardial infarction or severe hypertension.
3. Electrically pace the heart to decrease the QT interval
Electrical overdrive pacing with transcutaneous or transvenous stimulation has significantly improved capture. Some experts advocate that even when a patient has been controlled chemically, this should only be a bridge to electrical pacing4. The pacing mode should be set to asynchronous, also known as fixed rate, with the same target as above (90-110 bpm). Start at a high rate and titrate down.
4. Lidocaine may decrease the QT interval
Lidocaine is a type 1B antiarrhythmic that weakly blocks sodium channels and decreases the action potential and refractory period duration, resulting in shortened QT interval1. The literature base for the use of lidocaine for torsade storm is documented primarily by case studies in drug-induced TdP. Nonetheless, it is a readily available and familiar medication to most ED practitioners; whereas isoproterenol is unfamiliar and expensive and as such difficult to keep stocked in some EDs. A common protocol is below:

5. Phenytoin stabilizes the cardiac membrane
Phenytoin has been demonstrated in many case reports (this is not a complete review) to control medication refractory acquired TdP5–7. It is thought to work by stabilizing the cardiac membrane and prevents early after-depolarization from occuring5–7. In one study, a patient with ischemic heart disease and severely refractory TdP to magnesium, isoproterenol, and transvenous pacing was given 1000mg of IV phenytoin at 50 mg/min with resolution of her arrhythmia5. In another study, a patient with antipsychotic-induced TdP refractory to magnesium and isoproterenol was started on phenytoin infusion with normalization of her QTc within 24 hours6. In patients that are refractory to other interventions, phenytoin is a well-understood and safe medication to trial.

ECG Pointers:
- Torsades de Pointes is diagnosed by the combination of polymorphic ventricular tachycardia in addition to a prolonged QT-interval.
- Torsades mimics include severe hyperkalemia, coarse ventricular fibrillation, atrial fibrillation with Wolff-Parkinson-White, or Takotsubo cardiomyopathy
- In congenital long QT syndrome, EADs may be produced by high sympathetic output and thus treatment will focus on inhibiting sympathetic flow (beta-blockade).
- In acquired long QT syndrome, the cardiac membrane is unstable and prone to EADs, and beta-blockers will lengthen the QT interval and further increase risk of developing PVT.
- TdP is a ventricular tachycardia and the first line treatment for unstable patients is defibrillation.
- For stable patients with TdP initial treatment with 2 g IV magnesium pushed over ten minutes followed by an additional dose as needed and a 1-4 g/hr magnesium infusion is reasonable.
- Treatment for TdP storm includes magnesium and other interventions to decrease the QT interval and/or stabilize the cardiac membrane including isoproterenol, overdrive pacing, lidocaine, and phenytoin.
References:
- Thomas SHL, Behr ER. Pharmacological treatment of acquired QT prolongation and torsades de pointes. Br J Clin Pharmacol. 2016;81:420-427.
- Wilde AAM, Amin AS, Postema PG. Diagnosis, management and therapeutic strategies for congenital long QT syndrome. Heart. 2022;108:332.
- Farkas J. Torsade de pointes. EMCrit Project.
- Charlton NP, Lawrence DT, Brady WJ, Kirk MA, Holstege CP. Termination of drug-induced torsades de pointes with overdrive pacing. Am J Emerg Med. 2010;28:95-102.
- Omar HR, Sprenker C, Karlnoski R, Mangar D, Camporesi EM. The use of isoproterenol and phenytoin to reverse torsade de pointes. Am J Emerg Med. 2014;32:683.e5-683.e7.
- Vukmir RB, Stein KL. Torsades de pointes therapy with phenytoin. Ann Emerg Med. 1991;20:198-200.
- Mukhopadhyay S, Chakraborty P, Yusuf J, Goyal A, Tyagi S. Phenytoin in treatment of amiodarone-induced Torsades de pointes. Indian J Pharmacol. 2012;44:264.









