Authors: KC Collier, MD (EM Resident Physician, University of Vermont); Erica Lash, MD (EM Attending Physician, University of Vermont) // Reviewed by: Marina Boushra, MD (EM-CCM Physician, Cleveland Clinic Foundation); Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)
Case
A 62-year-old male with a history of diabetes, hypertension, and high cholesterol presents to the emergency department (ED) for back pain. He underwent an outpatient cardiac catheterization earlier in the day. Triage vitals show a blood pressure of 87/62 mm Hg and a heart rate of 110 bpm. His physical exam is significant for pale, diaphoretic skin, tachycardia, suprapubic tenderness, fullness, and severe lower back pain.
Introduction
Coronary artery disease (CAD) is the most common cause of death in the United States and the third leading cause of mortality worldwide.1 Consequently, it generates a significant economic burden, costing the US healthcare system hundreds of billions of dollars annually.1 Risk factors for CAD include non-modifiable factors such as family history and age and modifiable risk factors such as hypertension, hyperlipidemia, diabetes, and smoking. 1 The Atherosclerotic Cardiovascular Disease (ASCVD) score uses these risk factors to help predict an individual’s 10-year risk of a cardiac event or stroke.2 Similarly, the Thrombolysis in Myocardial Infarction (TIMI) risk score specifically stratifies for 14-day risk of all-cause mortality, new or recurrent MI, or severe recurrent ischemia requiring urgent revascularization.3
The varying degrees of CAD are described using the terms nonischemic heart disease (NIHD) and acute coronary syndrome (ACS), which is further stratified into unstable angina, Non-ST elevation MI (NSTEMI), and ST-elevation MI (STEMI) depending on disease severity.2 Notably, the nomenclature describing ACS is changing to “non-occlusive myocardial infarction (NOMI)” and “occlusive myocardial infarction (OMI).”4,5 This change in nomenclature reflects increasing recognition that the current STEMI criteria miss approximately one-fourth of all acute myocardial infarctions and other ECG findings exist that are better indicators of occlusive disease.4,5 The workup for CAD includes blood work, ECG, echocardiogram, and stress testing, but the gold standard diagnostic tool is cardiac catheterization.
Left Heart Catheterization Procedure
Left heart catheterization (LHC) is both a diagnostic and therapeutic intervention for CAD. Although one study demonstrated no difference in 5-year all-cause mortality between percutaneous intervention (PCI) and coronary artery bypass grafting (CABG), there are cases where cardiac catheterization and subsequent PCI is not the recommended course of treatment.6 For example, CABG is the intervention of choice for patients with diabetes who have triple-vessel disease.7 Additionally, PCI and CABG have different risk profiles that need to be considered in conjunction with the patient’s physiologic reserve.
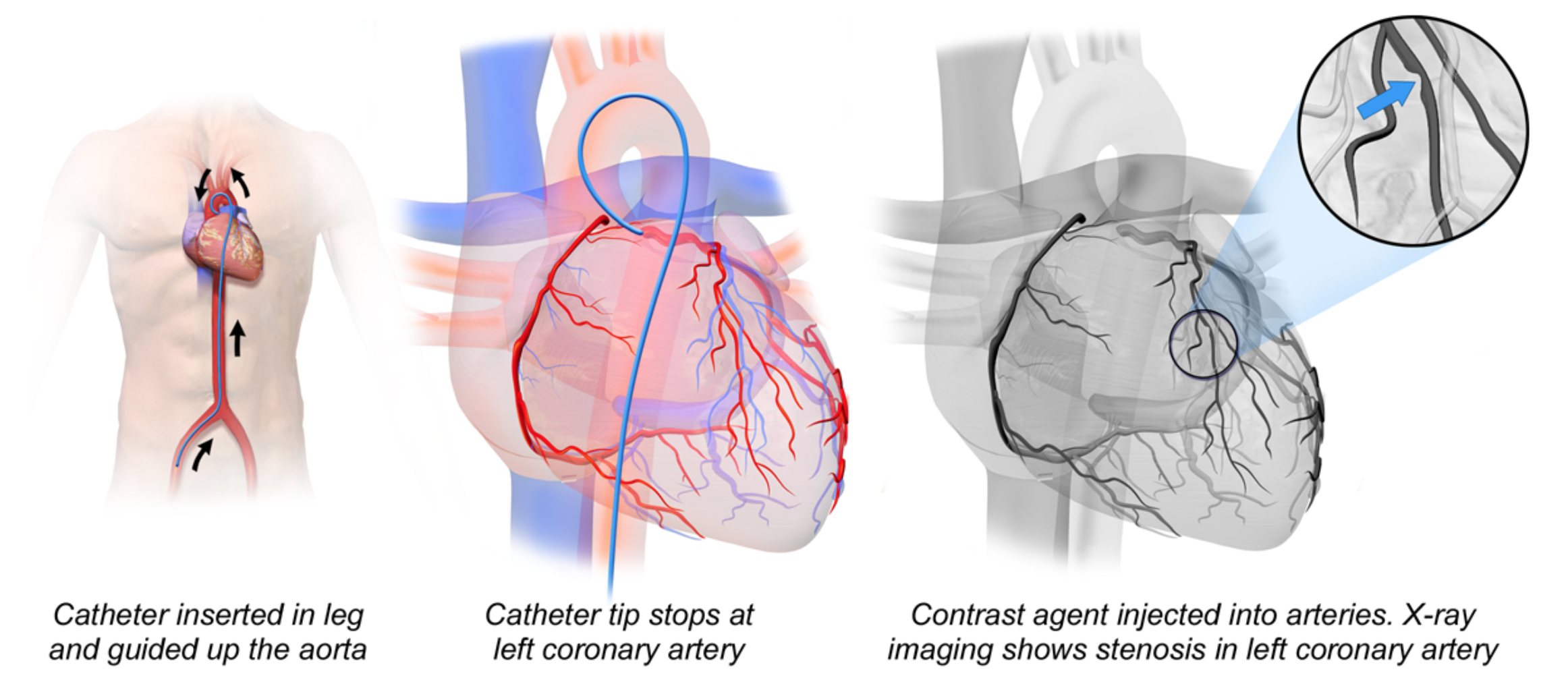
The procedure is performed by gaining arterial access through the radial or femoral artery. After access is obtained, a guiding catheter with a guidewire is inserted. The guidewire is then removed to so that atherosclerotic debris can flow out of the arterial system.8 Risk of thrombosis and embolism is decreased with preprocedural antiplatelet therapy (aspirin and a thienopyridine, i.e. clopidogrel or ticagrelor) and intraprocedural anticoagulation (i.e. heparin).8 Once in the ascending aorta, contrast dye is injected and fluoroscopy is used to advance the catheter and confirm its position in the coronary ostium.8 After determining the location of the lesion and appropriate device sizing, a stent mounted on an angioplasty balloon is placed over the guidewire. The device is then guided across the stenotic area and inflated, expanding the struts on the stent.8 The balloon and guide wire are removed and coronary angiography is performed to ensure patency of the stent and that revascularization has been achieved.8 This procedure can also be performed in saphenous vein grafts with some modifications.8
After catheter and wire removal, hemostasis at the access site can be achieved in a passive or active manner. Passive closure is accomplished by manual compression or with a compressive device that usually involves a pneumatic mechanism and incremental deflation.9 Active closure techniques include suture devices, plugs, and clips.9 Closure devices in femoral arteries enable faster hemostasis and allow patients to ambulate sooner, but manual compression remains the gold standard.9 Knowing the basics of this procedure is helpful for emergency physicians in anticipating complications.
Complications
Millions of cardiac catheterizations are performed every year.10 The risk of major complication–defined as stroke, pericardial effusion, unplanned CABG, or death–is low, with adverse events occurring in less than 1 per 1000 left heart catheterizations.10 The most common complications of LHC are vascular complications, including hematomas and retroperitoneal bleeding.11 The risk of a retroperitoneal bleed is significantly reduced with the use of trans-radial access (although nonzero) and even when a femoral approach is utilized, the risk of retroperitoneal bleed is less than 0.2%.11 Table 1 below lists common complications to consider in patients presenting following LHC.
Table 1: Procedure and device-related complications following left heart catheterization11, 12

Evaluation
History and physical examination:
If possible, a procedural report should be obtained to gain a better sense of what was done, what vessels were involved, what interventions were attempted, and if there were any complications. It is also important to ask about anticoagulation and antiplatelet medication use, both prior to and during the LHC.13
A complete physical exam should be performed with particular attention paid to the access site. It is important to evaluate the skin for swelling, ecchymosis, and other signs of bleeding, infection, or pseudoaneurysm formation. If an arteriovenous (AV) fistula or pseudoaneurysm has formed, it may be possible to appreciate a bruit on auscultation.11AV fistulas may also produce a pulsatile mass.11 If the bleeding is retroperitoneal, the patient may complain of back or flank pain and can display signs of shock and hemodynamic instability. In the event of embolization, it is important to assess for neurological deficits. Discoloration of the fingers and toes or livedo reticularis can be seen in cholesterol emboli.11
Lab work and Imaging:
Basic labs including a CBC, BMP, PT, PTT, and troponin should be obtained. Coagulation status should be evaluated, patients who have coronary artery disease and receive heparin have an increased (2-8%) risk for developing Type II heparin-induced thrombocytopenia (HIT).12 This usually occurs several days after heparinization, as it takes time for antibodies to form, although onset can be more rapid in patients who have been previously exposed to heparin.12 HIT is characterized by a 50% decrease in platelets regardless of the absence or presence of thrombus and is confirmed with HIT-antibody assays.12
EKG and bedside echocardiogram can help quickly assess for serious and life-threatening complications including pericardial effusion and wall motion abnormalities. If the physical exam demonstrates focal neurological deficits, patients should be evaluated for stroke. Most institutions have protocols that include routine imaging such as a head CT without contrast, CT angiography of the head and neck, and MRI head with and without contrast.13 Doppler color flow ultrasound is useful for evaluating for hematomas, pseudoaneurysms, and AV fistulas.12 CT with contrast and CTA are also appropriate imaging modalities to assess for other vascular injuries, in addition to retroperitoneal bleeding and dissection.
Management
Common vascular complications of LHC are pseudoaneurysm or AV fistula formation. Pseudoaneurysms are treated based on size. Pseudoaneurysms less than 3cm are likely to heal without intervention. Larger or symptomatic pseudoaneurysms may need to be addressed with an ultrasound-guided thrombin injection or surgery. 11 All AV fistulas require surgical repair.11
In patients with suspected bleeding complications, physicians should immediately type and screen the patient, obtain large bore access, and prepare to transfuse the patient. Depending on the time course of the bleeding, a CBC may be helpful to determine a baseline and may show low hemoglobin. Physicians should assess for overt signs of bleeding at the access site and apply direct pressure.13 Significant bleeding, such as retroperitoneal bleeds, should be managed with fluids, blood transfusion, reversal of anticoagulation, and potentially further invasive methods, such as coiling, stenting, or surgical repair of larger vessels.11 Either way, it is important to involve appropriate consulting services including cardiology, vascular surgery, and IR.
Case Conclusion
CTA of the abdomen and pelvis demonstrated a retroperitoneal hemorrhage. Direct pressure was applied and the patient was resuscitated in the emergency department. Cardiology was consulted. The patient was able to be treated with direct pressure and transfusion and was admitted for observation.14
Take Home Points
- Left heart catheterizations are common and complications are rare.
- Bleeding, including hematomas and retroperitoneal bleeding, are the most common complications.
- Ultrasound is helpful for assessing vascular injuries including hematoma, pseudoaneurysm, and AV fistula.
- CT with contrast or CTA is the imaging modality of choice to assess for retroperitoneal hemorrhage.15
- It is important to involve colleagues in cardiology, vascular surgery, and IR early in patient care, particularly if considering reversal of anticoagulation. An interdisciplinary discussion of risks and benefits of anticoagulation reversal is extremely important, given the anticipated side effect of increasing risk of in-stent thrombosis.
References:
1Brown JC, Gerhardt TE, Kwon E. Risk Factors For Coronary Artery Disease. 2022 Jun 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 32119297.
2Shahjehan RD, Bhutta BS. Coronary Artery Disease. [Updated 2022 Feb 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564304/
3Hess EP, Agarwal D, Chandra S, et al. Diagnostic accuracy of the TIMI risk score in patients with chest pain in the emergency department: A Meta-analysis. Canadian Medical Association Journal. 2010;182(10):1039-1044. doi:10.1503/cmaj.092119
4Aslanger EK, Meyers PH, Smith SW. STEMI: A transitional fossil in MI classification? Journal of Electrocardiology. 2021;65:163-169. doi:10.1016/j.jelectrocard.2021.02.001
5Pendell Meyers H, Bracey A, Lee D, et al. Accuracy of OMI ECG findings versus Stemi criteria for diagnosis of acute coronary occlusion myocardial infarction. IJC Heart & Vasculature. 2021;33:100767. doi:10.1016/j.ijcha.2021.100767
6Sabatine MS, Bergmark BA, Murphy SA, et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease: An individual patient data meta-analysis. The Lancet. 2021;398(10318):2247-2257. doi:10.1016/s0140-6736(21)02334-5
7Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for Coronary artery revascularization: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145(3). doi:10.1161/cir.0000000000001038
8Bangalore S, Bhatt DL. Right heart catheterization, coronary angiography, and percutaneous coronary intervention. Circulation. 2011;124(17). doi:10.1161/circulationaha.111.065219
9Rao SS, Agasthi P. Femoral Vascular Closure Devices After Catheterization Procedure. [Updated 2021 Dec 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557472/
10Al-Hijji MA, Lennon RJ, Gulati R, et al. Safety and risk of major complications with diagnostic cardiac catheterization. Circulation: Cardiovascular Interventions. 2019;12(7). doi:10.1161/circinterventions.119.007791
11Manda YR, Baradhi KM. Cardiac Catheterization Risks and Complications. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2021. PMID: 30285356.
12Tavakol M, Ashraf S, Brener SJ. Risks and complications of coronary angiography: A comprehensive review. Global Journal of Health Science. 2012;4(1). doi:10.5539/gjhs.v4n1p65
13Collier KC, Yang P. Left atrial appendage closure: Procedure basics, complications, and management. Left atrial appendage closure: procedure basics, complications, and management. http://www.emdocs.net/left-atrial-appendage-closure-procedure-basics-complications-and-management/. Published December 6, 2021. Accessed July 22, 2022.
14Kent KC, Moscucci M, Mansour KA, et al. Retroperitoneal hematoma after cardiac catheterization: Prevalence, risk factors, and optimal management. Journal of Vascular Surgery. 1994;20(6):905-913. doi:10.1016/0741-5214(94)90227-5
15 Verma N, Steigner ML, Aghayev A, et al. ACR Appropriateness Criteria® Suspected Retroperitoneal Bleed athttps://acsearch.acr.org/docs/3158181/Narrative/. American College of Radiology. Accessed October 10, 2022.







1 thought on “Left Heart Catheterization Complications: ED Presentations, Evaluation, and Management”
Pingback: Nerdfallmedizin.de - Nerdwoche vom 24.12.2022 – Xmas-Edition!