Author: Michael Folse, MD (EM Resident Physician, Parkland Memorial Hospital / UT Southwestern Medical Center) // Edited by: Alex Koyfman, MD (@EMHighAK) and Brit Long, MD (@long_brit)
It’s an arduous night in your forty-bed ED, and you just sat down to enter notes on the last three patients you’ve seen when one the technicians drops an EKG onto your desk. “STEMI, no STEMI,” She remarks as she briskly walks away. As you lament over this being your sixteenth patient with chest pain tonight, your eyes are immediately drawn to large ST-segment depressions across the anteroseptal leads, but with no ST-segment elevation. Being the astute clinician you are, you begin developing a broader differential as you put off your clerical duties just a little longer…
NSTEMI Background
As Non-ST Elevation Myocardial Infarction occurs on spectrum of illness, collectively referred to as Acute Coronary Syndrome (ACS), a proper introduction to ACS includes a discussion of its three entities: Unstable Angina (UA), NSTEMI, and STEMI.
Acute Myocardial Infarction or AMI encompasses both STEMI and NSTEMI. It can be divided into several categories: (type 1) infarction secondary to thrombosis, gradual or abrupt occlusion of the vessel, (type 2) cardiac damage due to a mismatch between cellular demand and coronary perfusion, (type 3) ischemia related to percutaneous coronary intervention, (type 4a) thrombosis of a coronary stent, and (type 4b) coronary artery bypass graft associated infarction.1 Take note of the key distinction between the NSTEMI (type 1) and NSTEMI (type 2). NSTEMI (type 1) indicates a new, thrombotic occlusion that requires immediate anticoagulation therapy. NSTEMI (type 2) is mainly a supply and demand mismatch that may require an alternative therapy based on the underlying etiology. For a visually appealing and concise depiction of the different legs of ACS, see, “Acute Myocardial Infarction,” by Anderson and Morrow in the New England Journal of Medicine. 2 To be classified as NSTEMI or STEMI, the patient has some form of cardiac biomarker release, signifying cardiac ischemia. In most hospitals in the United States, this is measured quantitatively by troponin I or troponin T levels. UA on the other hand may have EKG changes consistent with NSTEMI and many of the same symptoms, but, it cannot have a cardiac biomarker leak to be classified as such.
Classically, we think of NSTEMI as new myocardial ischemia, represented by increasing troponin, without ST-segment elevations, but often with contiguous ST-segment depressions. As a joint project in 2010, the American Heart Association (AHA) in collaboration with an number of other cardiac groups developed an expert consensus document outlining a strict definition for the EKG changes associated with NSTEMI: new horizontal or down-sloping ST depression >0.05 mV (0.5 mm) in two contiguous leads and/or T wave inversions equal or greater to 0.1mV (1 mm) in two continuous leads with prominent R wave or R/S ratio greater than 1.1
The diagnosis of NSTEMI in its earliest stages carries a large amount of uncertainty. The illness occurs on a spectrum, the severest of which often results in death or serious, life-altering morbidity. Therefore, it is vitally important to be cognizant of the myriad of other diagnoses that may have very different, time-critical, treatments. In an effort to provide a thorough but not overly-exhaustive discussion, only diagnoses that present with chest pain and rise in troponin will be considered. The following alternative diagnoses will be divided into three categories: immediate and critical, critical, and urgent.
Immediate and Critical
Posterior Myocardial Infarction
Consider the electrocardiogram below (Figure 1). The interpreter’s eyes are almost immediately drawn to the ST-segment depression in leads V2-V3. Without considering posterior MI, this EKG could be mistaken as a NSTEMI. Now, observe a repeat EKG with posterior leads (Figure 2). There are clear ST-segment elevations found throughout posterior leads V7-V9 warranting an immediate call to the catheterization laboratory. This is an isolated posterior STEMI, and it accounts for roughly 5% of all AMI cases.3
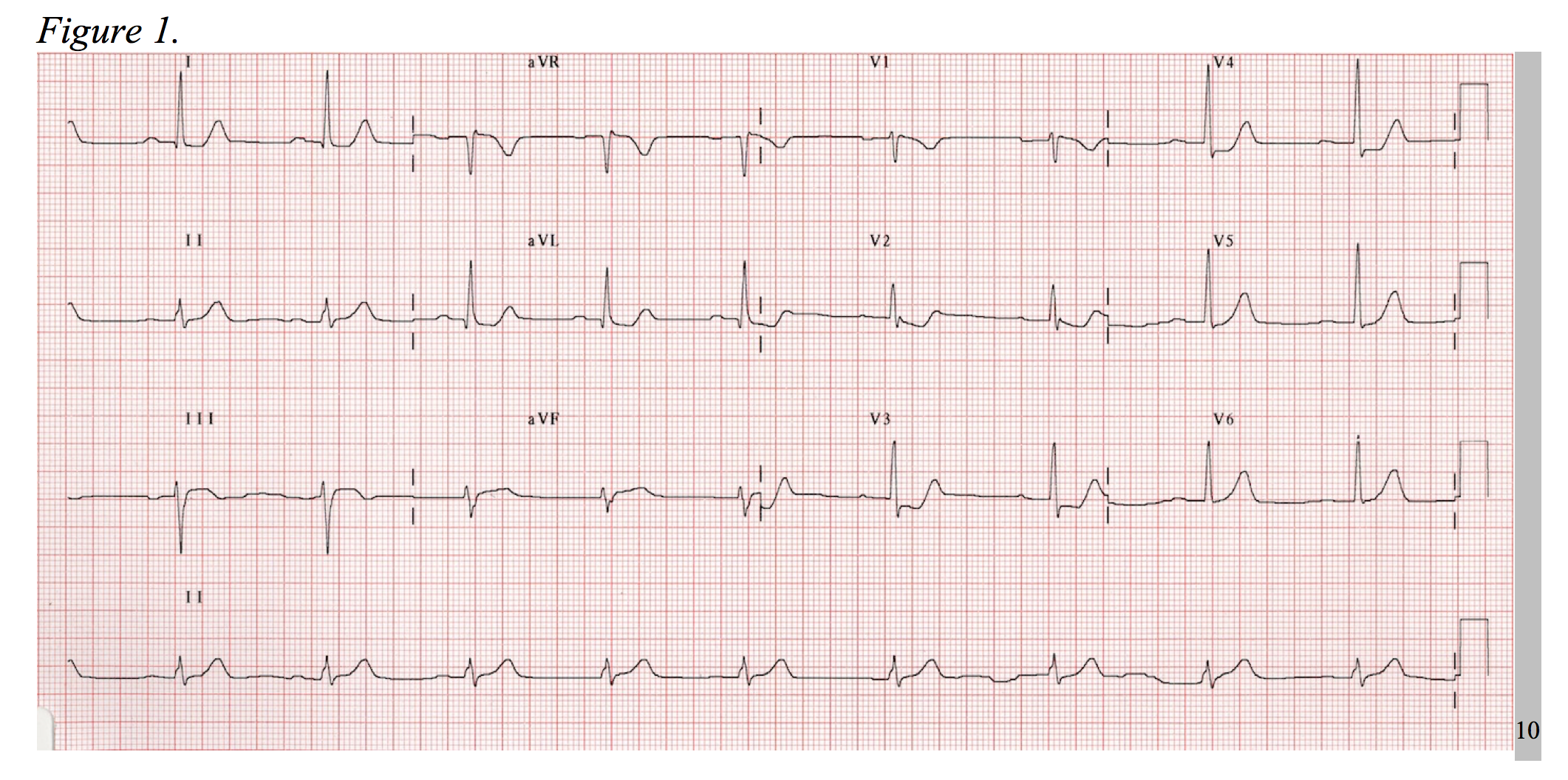
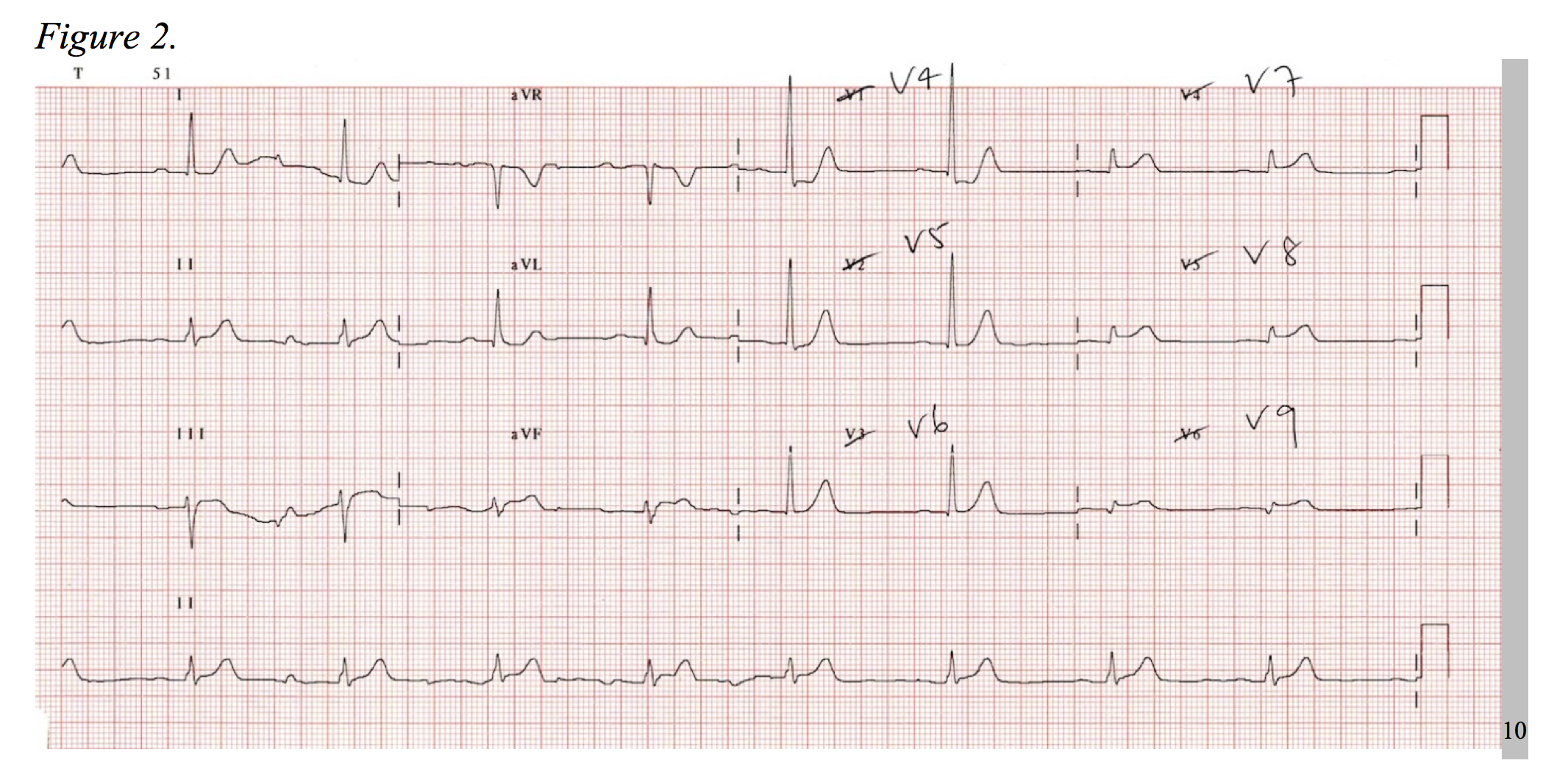
As a 15-lead EKG is not the standard triage screen for chest pain in most EDs, it is important to be aware of the signs of posterior MI on a standard 12-lead. Posterior infarctions generally have the following EKG findings in leads V1-V3: (1) wide R waves (> 0.04 seconds); (2) contiguous ST segment depressions with upright T-waves; (3) R/S ratios > 1 in V1 and V2.3,4 In other words, the ST-segment elevates in areas of transmural infarction and depresses in a direction opposite of this, while the T-wave elevates in the direction opposite of infarction. The length of the R-wave increases secondary to the loss of dorsally directed electrical forces depicted by a more positive anterior QRS-complex, and the R/S ratio is increased as reciprocal to a dorsally projected Q-wave.5
When diagnosing posterior MI by ST-segment elevation in the posterior leads, 0.5mm in two contiguous leads qualifies as an STEMI, as this increases the sensitivity from 58% to 94% when compared to 1mm of elevation.6
However, to complicate matters, multiple studies have shown angiographic evidence of MI in the setting of isolated posterior ST-segment elevations.7–9 In short, if there are signs of possible posterior MI or high suspicion for MI with a non-diagnostic EKG, perform an EKG with posterior leads. Even by modest standards, it can enhance the sensitivity of your investigation with little additional cost or time.
De Winter’s T-Waves / Proximal Left Anterior Descending Artery (LAD) Occlusion
In the right context, the following EKG (Figure 3) could be incorrectly interpreted as hyperkalemia. Considering the patient has chest pain, it could also be misjudged as subendocardial ischemia. However, the T-wave morphology depicted in this EKG is characteristic of a serious LAD occlusion and requires urgent catheterization.
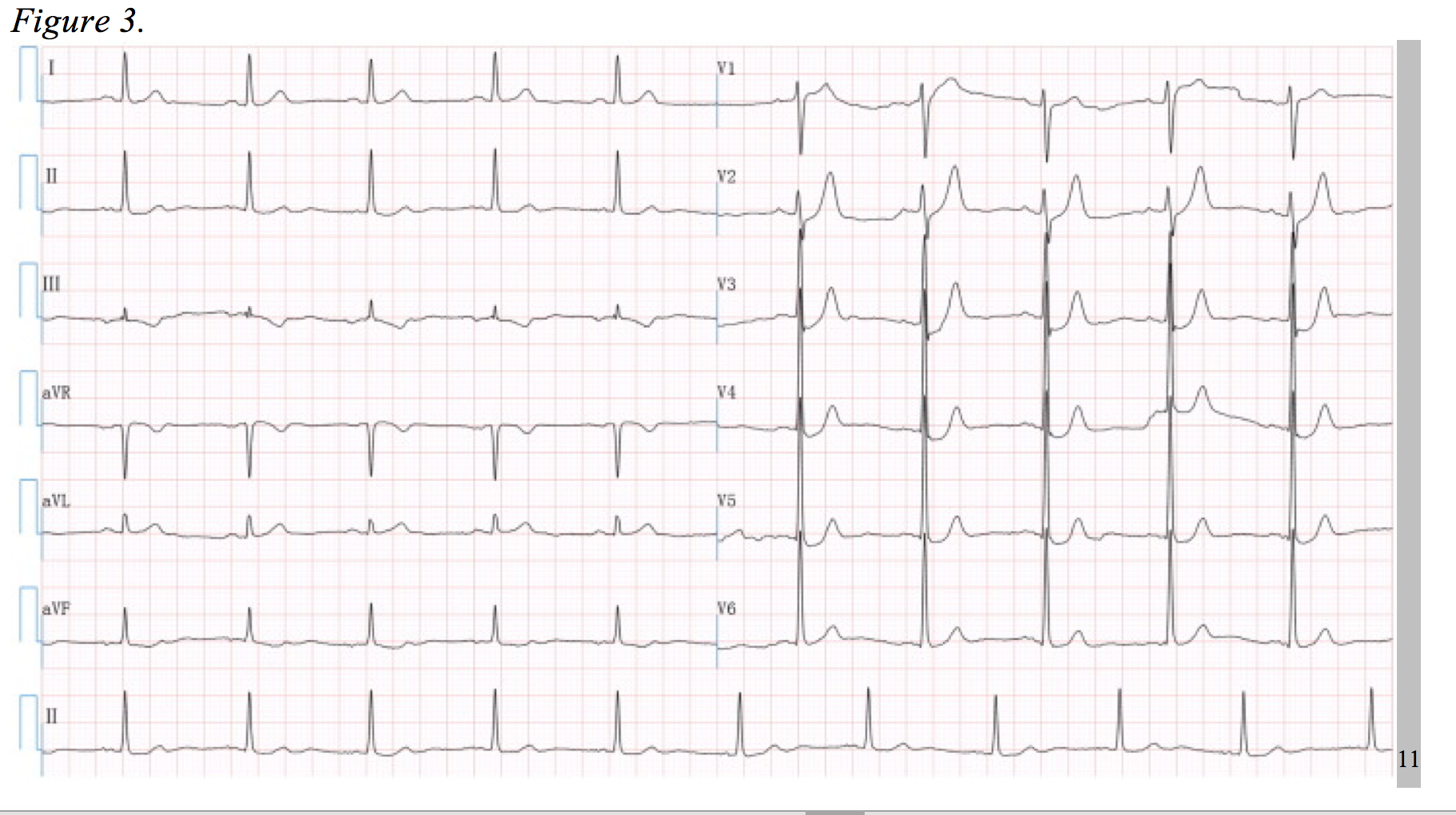
First coined by de Winter et al. in 2008, this pattern consists of a 1-3 mm upsloping ST-segment depression at the J-point throughout leads V1 to V6, becoming tall symmetrical T-waves. Additionally, there was a 1-2mm ST-segment elevation in lead aVR in all of De Winter’s cases; however, similar clinical scenarios have been documented without aVR elevation.11,12 In 2% of 1890 patients who underwent PCI for an LAD lesion, this distinct EKG pattern was evident and associated with significant cardiac ischemia. 11–13 The physiologic cause of such an EKG is unclear at this time, but the morbidity and mortality of this lesion is equivalent to a STEMI.11
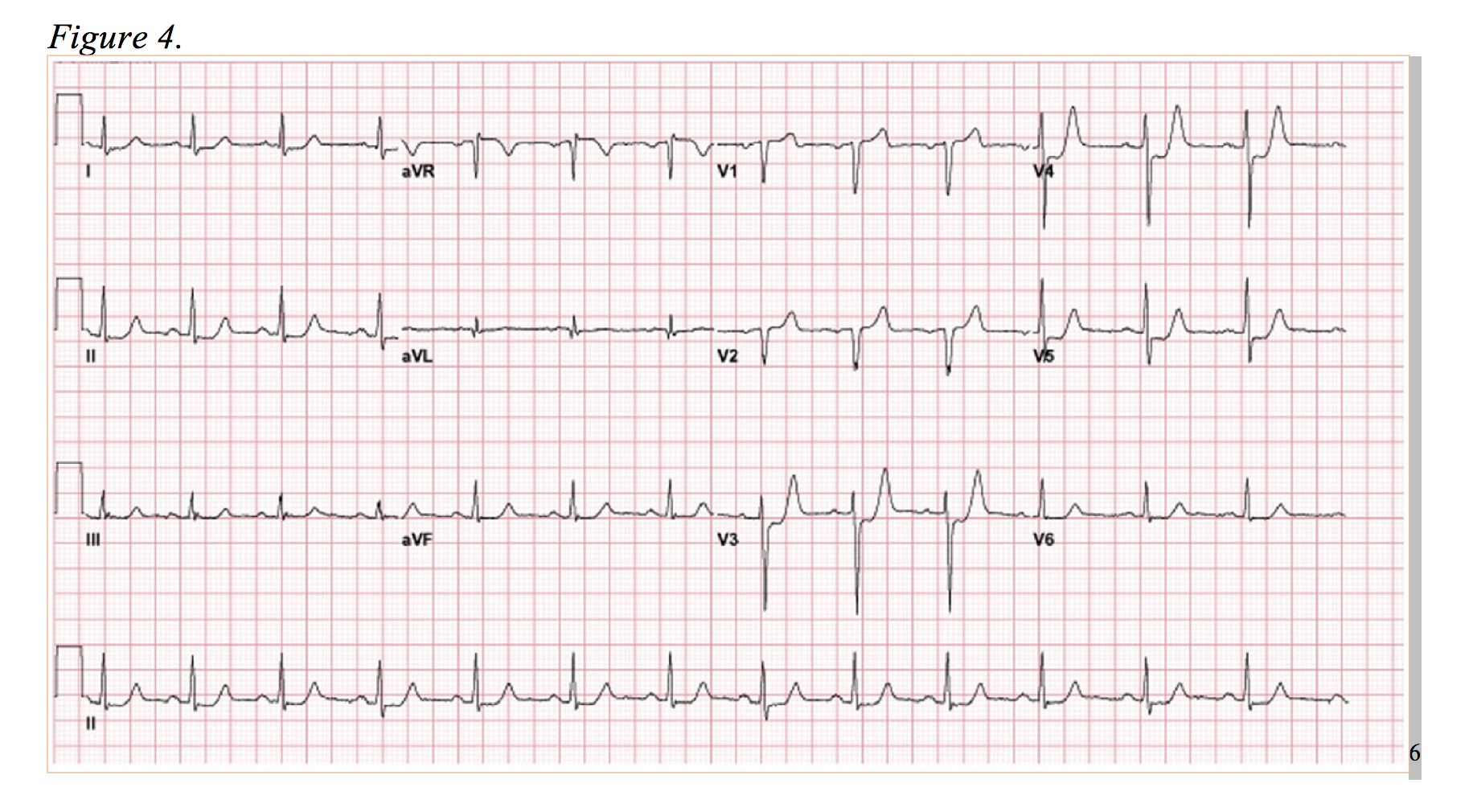
Now, observe Figure 4. The anterolateral ST-segment depressions are reminiscent of the posterior MI, and in this case, the ED physicians activated the catheterization lab on the presumption of a posterior MI. However, rather than a circumflex lesion, a complete occlusion of the LAD was discovered.6 In this EKG, there is evidence of anterior ST-segment depression with elevation in aVR. Presenting as a more subtle variant to de Winter’s T-waves, this is diagnostically difficult, but well documented occurrence.12,14 Of note, numerous studies correlate elevation of aVR as low as 0.5mm of ST elevation in an LAD occlusion to a more proximal lesion (proximal to S1) and greater in-hospital death rates.15,16
LMCA Occlusion/3-Vessel Disease
The final “immediate and critical” alternative diagnoses are those that present as global ST-segment depression and, often subtle, ST-segment elevation in aVR. Consider the EKG depicted in Figure 5. This EKG pattern generally represents one of two physiologic processes: cardiac stress in the setting of severe coronary artery disease or near-complete left main coronary occlusion.
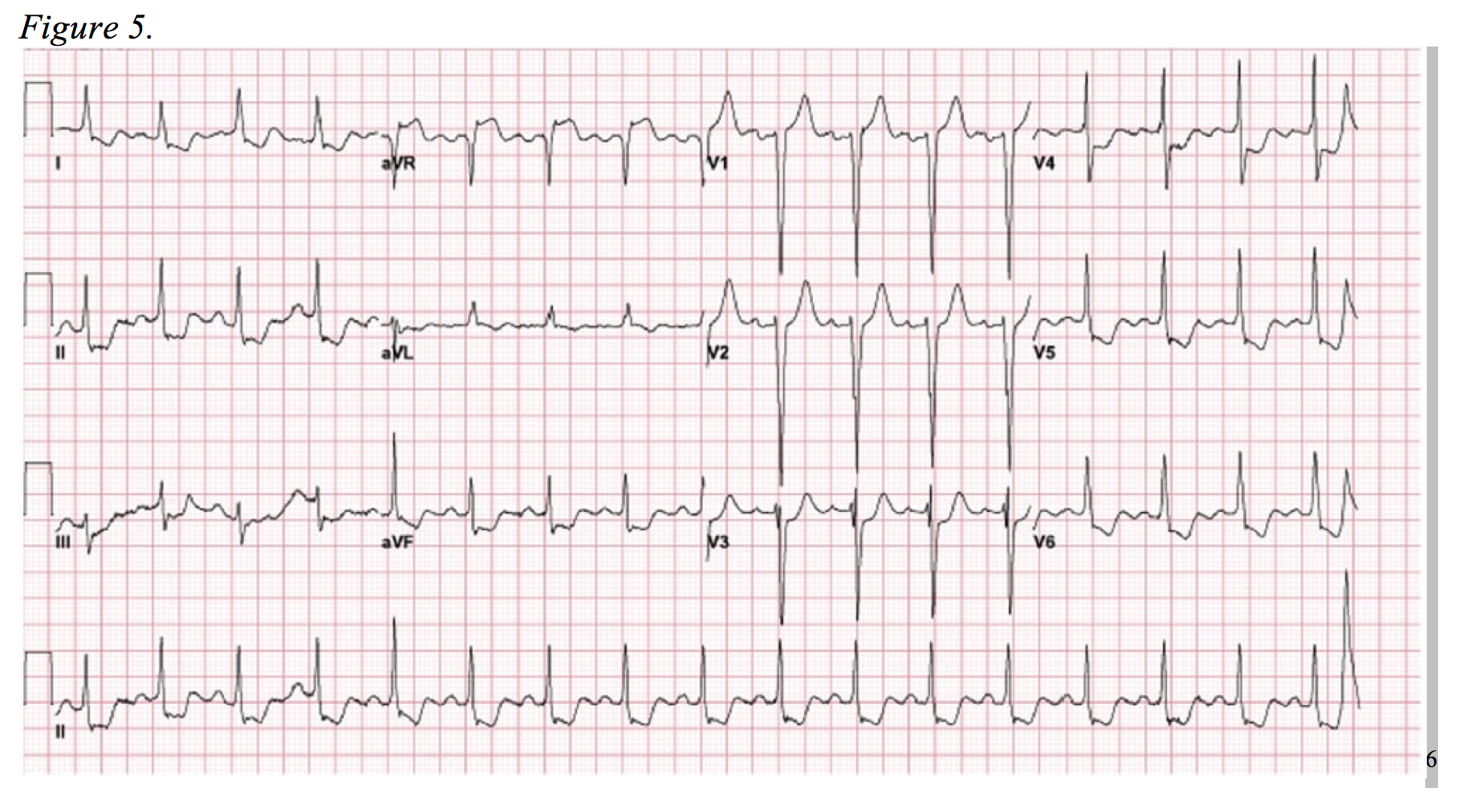
It is important to understand that complete left main coronary artery (LMCA) occlusion is a very uncommon infarct and occurs in less than 1.5% of all STEMI’s.17,18 Survival rarely follows a true full-occlusion, and it often presents with significant hemodynamic compromise and/or arrhythmia. 19,20 Furthermore, most true LMCA occlusions will display traditional STEMI criteria on EKG.21 However, there are a substantial number of documented cases in which diffuse ST-segment depression and isolated elevation in aVR are the only EKG signs apparent in this “STEMI equivalent.”7,19,22,23 Following suite, the ACCF/AHA guidelines for management of ST-segment elevation myocardial infarction included the aforementioned EKG pattern as a pattern require urgent cardiac catheterization in 2013.24
Accepting an NSTEMI pattern that can be seen in the setting of cardiac strain as a “STEMI equivalent” is a topic of debate.25 One of the studies used in developing the 2013 ACCF/AHA Guidelines by Jong et al. qualifies 50% stenosis as acute occlusion of the LMCA, which may be a clinical picture amendable to initial conservative medical management.22,25 Regardless, this EKG highlights the importance of the “forgotten lead:” aVR. A number of studies have documented the overwhelming disregard of this lead, even by experts, as it is often viewed as the reciprocal lead to the lateral leads that offers little additional information.26–28 Yet, aVR can lend the clinician pivotal insights into the diagnosis and management LMCA disease and severe three-vessel disease.
In a study of NSTEMI’s secondary to three-vessel or LMCA disease, aVR elevation > 1mm in lead aVR was 80% sensitive and 93% specific for either (1) >75% stenosis of the LM or (2) >90% stenosis LAD and >90% stenosis of right coronary artery and/or left circumflex. Roughly half of patients with an aVR ST-segment elevation greater than 1mm required urgent coronary artery bypass grafting.29 Prior to this study, Barrabés et al. found aVR ST-elevation to be significantly correlated with increased frequency of left main disease, three vessel disease, in-hospital death, recurrent ischemic events, and heart failure.30
Critical
The following “Critical” diagnoses are those in which medical decision making goes beyond sending the patient to the catheterization lab versus initiating noninvasive NSTEMI management. This is not to imply they require less-immediate action.
Pulmonary Embolus
While the majority (67%) of pulmonary embolisms (PE) present with pleuritic chest pain, Stein et al. found 4% of patients with PE to present with “angina-like” chest pain. Furthermore, many patients with NSTEMI can present atypically, impairing the clinician’s ability to differentiate between ACS and PE. EKGs offer little more information in comparison. The same study found 30% of those with PE to have normal EKG’s and 90% to be in sinus rhythm.31 There is a positive likelihood ratio of 3.7 associated with the classic S1Q3T3 finding, which is found in 8.8% of patients diagnosed with PE vs 3.3% in those without PE but with clinical suspicion. Tachycardia and incomplete right bundle branch block were 28.8% versus 15.7%, and 4.8% versus 2.8%, respectively.32 In regards to troponin elevation, Becattini et al. found it confers both an higher mortality rate (19.7% vs 3.7%) and adverse outcome rate (43.6% vs 14.7%) with an odds ratio of mortality at 7.95 (95% CI, 3.79 to 16.65) in the setting of troponin T elevation. 33 Therefore, troponin leak in the setting of PE offers information regarding prognosis but obfuscates diagnosis.
In an unstable presentation, sonographic signs of right heart strain such as McConnell’s sign and “D” sign can aid in diagnosis and should be correlated to the electrocardiographic signs discussed previously. Generally, around 35% of patients with an acute PE will show echocardiographic signs of right ventricular strain.34 A history of cancer, previous PE, previous deep vein thromboses (DVT), long distance travel, oral contraceptive use, and recent surgery should also be ascertained.
Fortunately, computed tomography angiography (CTA) and V/Q scan serve as effective modalities of diagnosis in stable patients, and a d-dimer level can be useful in ruling out PE in low-probability cases that are Pulmonary Embolism Rule-Out Criteria (PERC) positive. In short, the diagnosis and treatment of PE is worthy of its own discussion; but, suffice it to say that CTA, is a relatively sensitive and specific tool. The PIOPED II study, using mainly 4-section scanners developed prior to 2006, found a sensitivity and specificity of CTA to be 83% and 96%, respectively. However, with the advent dual-source CT and 64-section scanners, the sensitivity of PE detection has further increased.35,36 For many clinicians, detection has improved to the degree of questioning whether some pulmonary emboli (sub-segmental pulmonary emboli) are even clinically relevant; and in 2016, The American College of Chest Physicians began advising against treating sole sub-segmental pulmonary emboli in low-VTE risk patients as a Grade 2C recommendation.37,38
Aortic Dissection
In a review of 235 patients diagnosed with aortic dissection, Spitwell et al. found the clinician to have the correct clinical impression of aortic dissection in 68%, 51%, and 73% for DeBakey type I, II, and III dissections, respectively, after review of a corresponding patient’s medical history, physical exam, ECG, and radiograph by an attending physician.
The diagnosis of aortic dissection is challenging, and to give anticoagulation or thrombolytic agents to a dissecting patient, although not unthinkable, is potentially life-threatening. Keep in mind that an acute aortic dissection (AAD) may present with chest pain plus another pain and/or another vascular or neurologic sign; back pain (53.2%), abdominal pain (29.6%), migrating pain (16.6%), pulse deficit (15.1%), cerebrovascular accident (4.7%). Additionally, search for a murmur aortic insufficiency (31.6%), and determine if the chest pain is tearing in nature (50.6%).39 Chest radiographs have sensitivities between 60% and 90%, lower at more proximal locations along the aorta.40,41 Considering only elements of physical exam and radiography, Kodolitsch et al. found that 96% of patients with acute dissections will have at least one of the following: immediate onset of chest and/or back pain, pain described as tearing or ripping; loss of carotid pulse or extremity pulse, and/or blood pressure differentials >20 mmHg systolic; mediastinal or aortic widening on radiograph.42
Kosuge et al., discovered that while 30% had a normal EKG at time of diagnosis, about 47% of patients in a cohort of 233 had ST depressions and/or T-wave depressions, which was closely associated with severe hypertension, positive troponin, pericardial effusion, and cardiac tamponade.43 Likely as product of type II ischemia secondary to severe hypotension, hypertension, or aortic insufficiency, one meta-analysis demonstrated an association with elevated troponin and increased mortality.44 Like troponin elevation in PE, troponin and ST-depression serve as obscuring signals with hints of mortality.
When imaging a suspected aortic dissection, choose a modality appropriate to hemodynamic stability of the patient and the urgency of the situation. Transesophageal echocardiography (TEE) if often utilized in more acute situations as TEE can yield a positive diagnosis in only minutes. The caveats is that it is user proficiency dependent, and the hospital must have interpreters and operators readily available.41,45,46 Not surprisingly, the majority of aortic dissections are initially imaged by computed tomography (CT).39 Fortunately for emergency physicians, computed tomography touts a similar sensitivity and often beats TEE in regards to specificity, even in thoracic dissections (87.1% vs 76.9%).47 The specificity of TEE has been observed to fall when the dissection is in more distal locations.46 In a minority of cases, transthoracic echocardiography is employed. Although it lacks the sensitivity of the other modalities, it is relatively specific when performed by experienced operators.47 If time permits, it may be useful to perform a bedside echocardiogram. Regardless, if aortic dissection is highly suspected, another imaging study is warranted. The average number of imaging modalities to diagnosis in the International Registry of Aortic Dissection was 1.8.39 However, as in low-risk patients being considered for PE, the days of ordering an advanced imaging in otherwise low-risk patients being considered for AD may soon come to an end. D-dimer has been suggested as a rule out in very low risk patients, though this is controversial. A recent systematic review and meta-analysis suggests that the posttest probability of an aortic dissection to be 0.3% following a negative d-dimer (< 0.5 ug/ml) and a low-risk score (0/3) on a risk stratification scoring system developed by the AHA.48,49 Regardless of one’s interpretation of this data, bear in mind that the mortality rate of AAD has been estimated to be almost 30%.39 Thus it is important to keep a high index of suspicion, be vigilant of other signs of vascular compromise when evaluating a patient with chest pain, and seek advanced imaging when necessary.
Urgent
The following diagnoses labeled as “urgent” afford the clinician more time in differentiating the disease presentation, the initial management is equivalent to NSTEMI in the emergency department, and/or discrepancies between the disease process and NSTEMI are more distinct.
Spontaneous Coronary Artery Dissection
At approximately 800 cases per year in the United States and an estimated 0.2% of ACS cases, spontaneous coronary artery dissection (SCAD) is rare diagnosis that could potentially mimic the presentation of an atherosclerotic NSTEMI.50,51 While the majority are likely to present as STEMI’s, in a single center study of 87 patients, NSTEMI presentations accounted for 44% SCAD.50 Although the full mechanism of this disease is not completely understood, it has been postulated that it involves either an intimal tear or a bleeding vasa vasorum that propagates an expanding false lumen as in an aortic dissection, compressing the true lumen.52 In the largest known cohort of nonatherosclerotic SCAD, 100% had troponin-I elevations, 92.3% were women, 72% had fibromuscular dysplasia, and 48% were under the age of 50.53 In an additional publication, Saw et al., reported SCAD as the culprit in nearly a quarter of women under the age of 50 with MI.54 As emergency physicians, our role in early management remains unchanged, but the subject highlights the importance of recognizing the possibility of true cardiac ischemia in young females without typical cardiovascular risk factors. Consider sending a troponin in younger female patients with concerning chest pain.
Takotsubo Cardiomyopathy
Also referred to as apical heart ballooning syndrome and stress cardiomyopathy, Takotsubo Cardiomyopathy occurs at rate of 1% to 2% of patients presenting with ACS.55,56 Takotsubo, the Japanese term “octopus pot,” relates to the morphology secondary to mid-cavity to apical dysfunction of the heart during the systolic phase. The mechanism of this dysfunction is not fully understood but likely relates to catecholamine stunning of the myocardium and direct cardiac toxicity by elevated levels of circulating catecholamine.55,57 Of 1,750 patients diagnosed with Takotsubo Cardiomyopathy, Templin et al., found 36% to have recent and significant physical stressor, 27.7% to have a recent significant emotional stressor, and 7.8% to have both. Of note, almost 90% of the European cohort were female, and 55.8% had a recent diagnosis of a neurologic or psychiatric disorder.58
The clinical picture of stress cardiomyopathy is nearly indistinguishable from ACS. The predominant symptom at admission is chest pain in almost 80% of cases. Troponin levels are elevated in nearly 90% of patients, while brain natriuretic peptide is elevated in about 80%. ST-segment elevation is seen about 44% of the time, and ST-segment depression at roughly 8%.58 However, even with a high suspicion of Takotsubo Cardiomyopathy, ACS should be considered until proven otherwise.
Myocarditis and Pericarditis
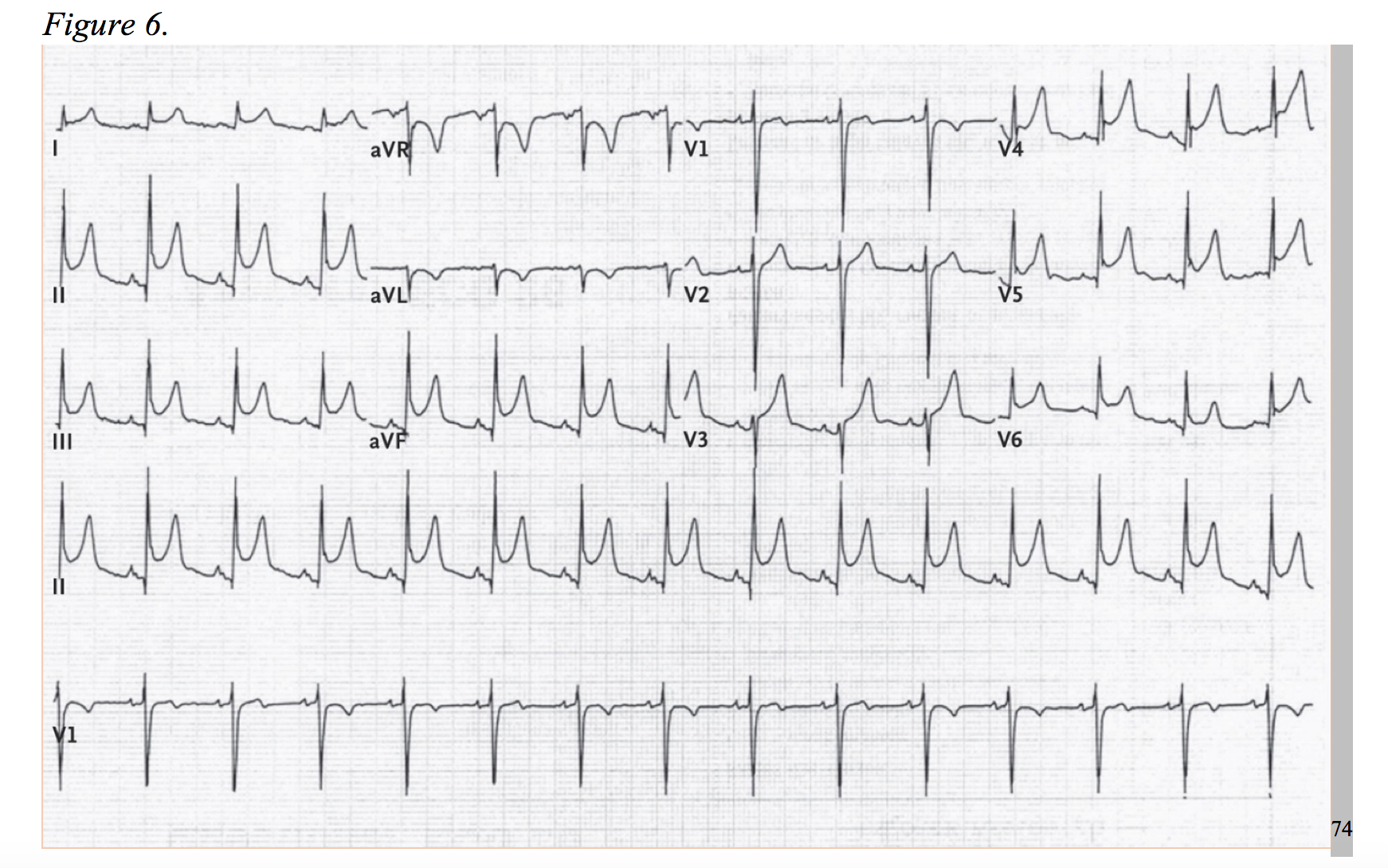
Myocarditis is caused by a number of agents: viral infections (commonly, but not limited to adenovirus or enterovirus), bacterial infection, autoimmune response, toxins, or systemic illness.59 The etiologies, although generally viral, are vast. In hand with the heterogeneity of causality, the clinic presentation is just as variable, ranging from subclinical to sudden death. In the European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases, the majority (71.7%) presented with dyspnea, 31.9% with nonspecific chest pain, and 17.9% with arrhythmic events.60 Myocarditis cases with clinic presentations identical to ACS coupled with electrocardiograms with focal ST-elevations, and at times, ST-depressions, are well documented.59,61–65 Roughly 30% to 60% of cases will present with troponin elevations.66,67 Short of sudden death, some patients will progress to heart failure. Myocarditis accounted for 9-10% of initially unexplained cardiomyopathy in two studies.68,69 Pericarditis, inflammation of the epicardium, shares similar etiologies myocarditis, but the diagnostic incidence occurs at a higher rate, roughly 5% of non-ischemic chest pain admissions.70,71 Additionally, nearly one-third of pericarditis cases are associated with myocarditis and will potentially cause a troponin leak.72,73 The EKG of pericarditis typically demonstrates diffuse ST-elevations in the acute phase (Figure 6), but a percentage of cases project negative T-waves before completely normalizing. Cases of focal ST-elevations have been documented; and therefore, pericarditis is more likely to present as a STEMI-mimic but has the potential to act as an NSTEMI-mimic in the right setting. In addition to typical EKG findings, an elevated C-reactive protein (~75% of cases), white blood cell count, and a friction rub on examination can aid in differentiating pericarditis from ACS.73,74
Hypertrophic Cardiomyopathy (HCM) & Hypertrophic Obstructive Cardiomyopathy (HOCM)
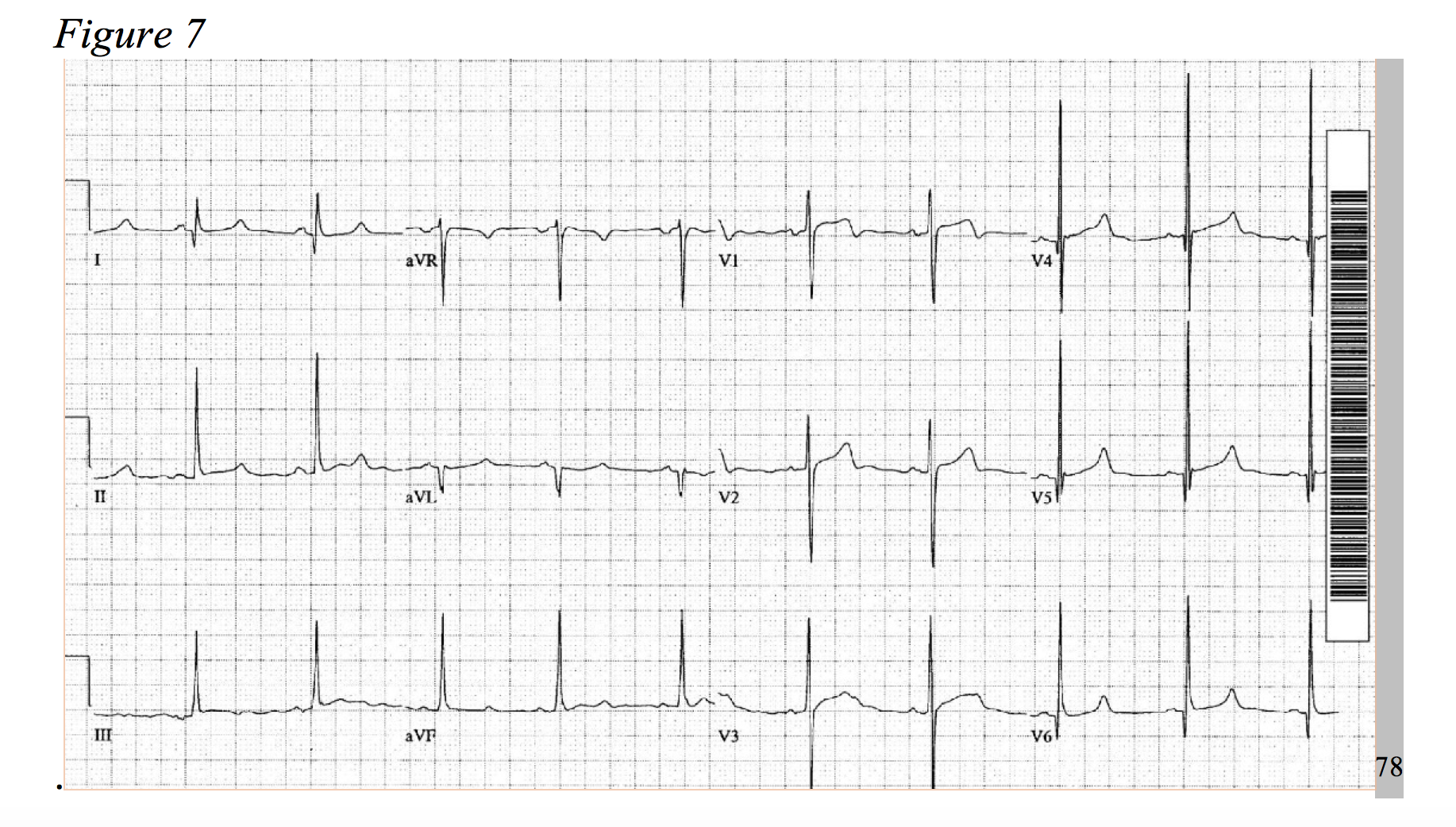
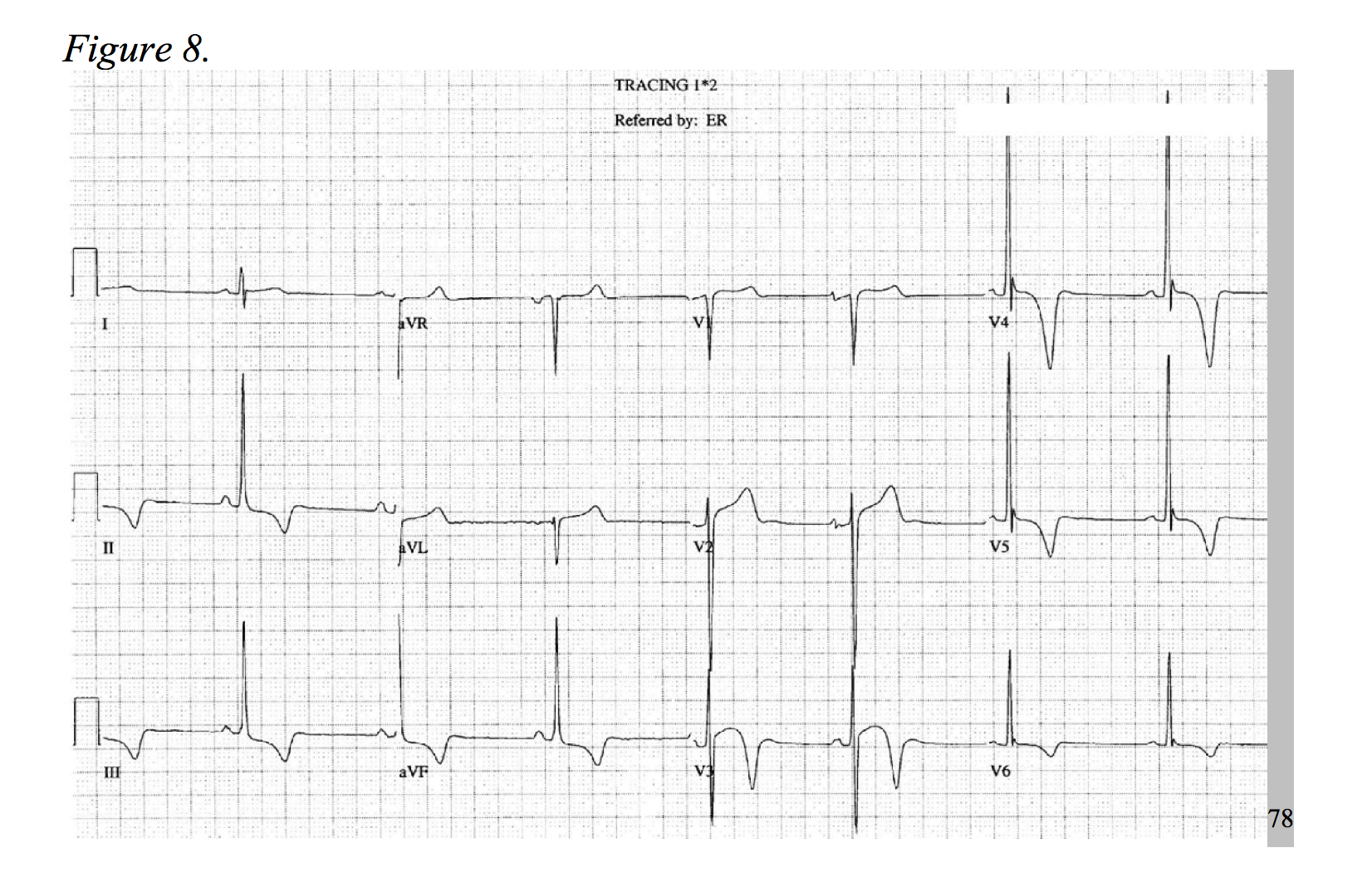
At a prevalence of about 1 in every 500 adults, hypertrophic cardiomyopathy is a rather common disease, causing an increase of cardiac muscle mass of the ventricular septum.75,76 Typical symptoms include dyspnea, chest pain, syncope, and palpitations.77 HOCM, the most common cause of sudden death in young patients, more frequently presents as chest pain in younger patients.78 The physiology of the disease is multifactorial and includes dynamic left ventricular outflow tract obstruction, diastolic dysfunction, mitral regurgitation, and myocardial ischemia.79 Considering the development of hypertrophied cardiac myocytes and obstructive hemodynamics, supply-demand ischemia can develop and may present as focal ST-segment depressions under stress.78,80 As an association of disease severity, one study found troponin elevation over 0.04ng/ml in nearly one third of HOCM patients.81 An atherosclerotic NSTEMI-mimic in a presentation HOCM is possible; but, the patient will likely be younger and lack typical risk factors for ACS, while the chest pain is classically exertional in nature. As only 10% of patients with hypertrophic cardiomyopathy present with a normal EKG, identify signs of significant left ventricular hypertrophy, left axis deviation, prominent septal Q-waves, and in some cases deeply inverted T-waves (Figures 7 & 8).77,78,82
Aortic Stenosis (AS) & Aortic Insufficiency (AI)
Like HOCM, aortic valve disease can mimic an atherosclerotic NSTEMI via cardiac strain. The typical symptoms are exertional dyspnea, but patients can present with angina, syncope, and/or heart failure. Hypertrophy of the ventricles in response to increased afterload leads to decreased left ventricle compliance, increased O2 demand, lower coronary perfusion, and in late stages, systolic dysfunction.83 Considering that the average age of even asymptomatic AS is around 60 years old and about half of patients have some level of coronary artery disease, the presentations are variable and are often a hybrid of ACS and AS.84,85 In a cohort of 123 adults with asymptomatic AS, nearly 70% produced focal ST-segment depressions when performing an exercise stress test.85 There is a high likelihood of severe aortic stenosis presenting as an NSTEMI in the setting of cardiac stress. In addition to searching for left ventricular strain on EKG, search for a long crescendo-decrescendo murmur on physical exam and a weak, slow-rising pulse.83
Similarly, aortic insufficiency will present with angina, but at a much lower rate. More classically, dyspnea and symptoms associated with left sided heart failure occur. The pathophysiology of AI derives from an incompetent aortic valve leading to volume overload of the left ventricle, mainly eccentric hypertrophy, and systolic dysfunction. Angina in AI, however, is predominantly caused by a decrease in diastolic pressure of the aorta with an increased in diastolic pressure within the left ventricle, limiting coronary runoff from the aorta.86 In severe cases, stress patterns (ST depressions), troponin elevation, and an NSTEMI-like picture could occur.87,88 When evaluating a patient with suspicion of AI, search for a history of collagen disease and aortic root disease, and on physical examination, a wide pulse pressure and a diastolic blowing murmur across the left sternal border.86
Congestive Heart Failure (CHF)
The emergency physician is well acquainted with the signs and symptoms of CHF: peripheral edema, jugular venous distension, pulmonary rales, dyspnea on exertion, orthopnea, and occasionally chest pain. B-type natriuretic peptide (BNP) is often elevated in proportion to the extent of acute heart failure. Considering that coronary artery disease is the largest cause of CHF, there is a significant amount of overlap in the symptomatology and laboratory findings in acute presentations of CHF and type 1 NSTEMI cases. Three-fourths of patients in acute heart failure admitted to the hospital have a detectable level of troponin (TnI >0.4 ng/ml or TnT > 0.01ug/l).89 The cause of elevated troponin in CHF is multifactorial: increased wall stress, epicardial CAD, oxidative stress, altered calcium handling, inflammatory cytokines, and neurohormonal activation. Interestingly, not all are associated with myonecrosis.90 Therefore, patients with elevated troponin require careful chart review of previous levels of troponin, BNP or NT-proBNP levels, those levels in relation to troponin levels, previous weights relative to current weight, and troponin relative to glomerular filtration rate in the setting of chronic kidney disease or end stage renal disease.91–93 A general sense of cardiac function can be gained by a quick bedside echocardiogram, and assessment of volume overload and pulmonary edema can be performed by searching for B-lines (> 3 per view in one of eight thoracic fields).94,95 This must also be correlated to the clinic presentation. If there is still clinic uncertainty, coronary angiography is warranted, and the patient should be treated as a type 1 NSTEMI.
Pulmonary Artery Hypertension (PAH) & Chronic Obstructive Pulmonary Disease (COPD)
With similar pathophysiology of myocardial injury in PE, AS, AI, and CHF, pulmonary artery hypertension can cause elevated troponin levels and chest pain. One study estimates 14% of clinical stable patients with chronic PAH to have an elevated troponin T, and numerous studies have correlated elevated troponin with increased mortality as in CHF.96–99 In addition to the pathophysiology associated with pulmonary hypertension, troponin elevation in COPD is triggered by increased respiratory work, increased intrathoracic negative pressures, and increased left ventricular afterload. Multiple studies have also cited an elevated troponin as prognostic factor for increased mortality.91,100–103 Fortunately, COPD exacerbations are unlikely to present with angina-like chest pain, and the patient will likely have a consistent history and physical exam. However, diagnostic confusion may arise as an end-expiratory wheezing can sound like a cardiogenic wheeze, especially in the setting of reduced lung expansion. In cases such as this, consider a sonographic examination of the lung fields and heart in search of depressed cardiac motion and evidence of pulmonary edema. But, beware that intestinal fibrosis may also present with B-lines.94,95 Correlate these findings to pulmonary auscultation, current and previous chest x-ray findings, and possibly BNP, NT-proBNP, and/or troponin levels. The patient’s response to intervention should further elucidate the underlying cause, but always be vigilant of ACS.
Summary
- Type 1 NSTEMI involves a new, partial occlusion of the cardiac vessel. Type 2 NSTEMI involves a supply-demand deficiency.
- Always check for reciprocal ST-segment elevation when observing ST-segment depression.
- Posterior MI diagnosis requires ST-segment elevation of 0.5mm or greater.
- Upsloping ST-segment depressions with tall T-waves through V1-V6 is a STEMI equivalent.
- The American Heart Association recognizes aVR ST-segment elevation in the setting of anterolateral ST-segment depression as an indication for emergent cardiac catheterization.
- PE and dissection are important mimics of NSTEMI.
- Consider inflammatory markers in suspected cases of myocarditis and pericarditis.
- Differentiation of NSTEMI-mimics that involve a physiologic increase in post-left/right ventricular afterload (HOCM, AI, AS, PAH, COPD) can generally be made by careful examination of the patient’s medical history and clinical presentation.
See the following video for a short visual of some of the sonographic findings discussed in this article: https://youtu.be/rITbURahy2c
References / Further Reading
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third Universal Definition of Myocardial Infarction. Circulation. 2012;126(16):2020-2035. doi:10.1161/CIR.0b013e31826e1058.
- Anderson JL, Morrow DA. Acute Myocardial Infarction. N Engl J Med. 2017;376(21):2053-2064. doi:10.1056/NEJMra1606915.
- Thomas JJ, Brady WJ. Acute Coronary Syndrome. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Philadelphia, PA: Elsevier; 2018:891-928.e4. https://www-clinicalkey-com.foyer.swmed.edu/#!/content/book/3-s2.0-B9780323354790000684?scrollTo=%23hl0000949.
- Perloff JK. The Recognition of Strictly Posterior Myocardial Infarction by Conventional Scalar Electrocardiography. Circulation. 1964;30(5):706-718. doi:10.1161/01.CIR.30.5.706.
- van Gorselen EOF, Verheugt FWA, Meursing BTJ, Oude Ophuis AJM. Posterior myocardial infarction: the dark side of the moon. Neth Heart J. 2007;15(1):16-21.
- Hennings JR, Fesmire FM. A new electrocardiographic criteria for emergent reperfusion therapy. Am J Emerg Med. 2012;30(6):994-1000. doi:10.1016/j.ajem.2011.04.025.
- Wung S-F, Drew BJ. New electrocardiographic criteria for posterior wall acute myocardial ischemia validated by a percutaneous transluminal coronary angioplasty model of acute myocardial infarction. Am J Cardiol. 2001;87(8):970-974. doi:10.1016/S0002-9149(01)01431-X.
- Agarwal JB, Khaw K, Aurignac F, LoCurto A. Importance of posterior chest leads in patients with suspected myocardial infarction, but nondiagnostic, routine 12-lead electrocardiogram. Am J Cardiol. 1999;83(3):323-326. doi:10.1016/S0002-9149(98)00861-3.
- Schmitt C, Lehmann G, Schmieder S, Karch M, Neumann F-J, Scho¨mig A. Diagnosis of Acute Myocardial Infarction in Angiographically Documented Occluded Infarct Vessel. Chest. 2001;120(5):1540-1546. doi:10.1378/chest.120.5.1540.
- Posterior Myocardial Infarction – Life in the FastLane ECG Library. LITFL • Life Fast Lane Med Blog. September 2011. https://lifeinthefastlane.com/ecg-library/pmi/. Accessed August 21, 2017.
- de Winter RJ, Verouden NJW, Wellens HJJ, Wilde AAM. A New ECG Sign of Proximal LAD Occlusion. N Engl J Med. 2008;359(19):2071-2073. doi:10.1056/NEJMc0804737.
- Zhao Y-T, Chia-Chen C. Impending anterior myocardial infarction: de Winter syndrome. Am J Emerg Med. 2016;34(12):2450-2451. doi:10.1016/j.ajem.2016.09.019.
- Verouden NJ, Koch KT, Peters RJ, et al. Persistent precordial “hyperacute” T-waves signify proximal left anterior descending artery occlusion. Heart. 2009;95(20):1701-1706. doi:10.1136/hrt.2009.174557.
- Gul EE, Nikus KC. An unusual presentation of left anterior descending artery occlusion: significance of lead aVR and T-wave direction. J Electrocardiol. 2011;44(1):27-30. doi:10.1016/j.jelectrocard.2010.08.009.
- Engelen DJ, Gorgels AP, Cheriex EC, et al. Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J Am Coll Cardiol. 1999;34(2):389-395. doi:10.1016/S0735-1097(99)00197-7.
- Aygul N, Ozdemir K, Tokac M, et al. Value of lead aVR in predicting acute occlusion of proximal left anterior descending coronary artery and in-hospital outcome in ST-elevation myocardial infarction: an electrocardiographic predictor of poor prognosis. J Electrocardiol. 2008;41(4):335-341. doi:10.1016/j.jelectrocard.2008.02.025.
- Kurisu S, Inoue I, Kawagoe T, et al. Electrocardiographic features in patients with acute myocardial infarction associated with left main coronary artery occlusion. Heart. 2004;90(9):1059-1060. doi:10.1136/hrt.2003.026799.
- Zoghbi GJ, Misra VK, Brott BC, et al. ST ELEVATION MYOCARDIAL INFARCTION DUE TO LEFT MAIN CULPRIT LESIONS: PERCUTANEOUS CORONARY INTERVENTION OUTCOMES. J Am Coll Cardiol. 2010;55(10):A183.E1712. doi:10.1016/S0735-1097(10)61713-5.
- Hori T, Kurosawa T, Yoshida M, Yamazoe M, Aizawa Y, Izumi T. Factors Predicting Mortality in Patients after Myocardial Infarction Caused by Left Main Coronary Artery Occlusion. Jpn Heart J. 2000;41(5):571-581. doi:10.1536/jhj.41.571.
- Ward DE, Valantine H, Hui W. Occluded left main stem coronary artery. Report of five patients and review of published reports. Heart. 1983;49(3):276-279. doi:10.1136/hrt.49.3.276.
- Yamaji H, Iwasaki K, Kusachi S, et al. Prediction of acute left main coronary artery obstruction by 12-lead electrocardiography. J Am Coll Cardiol. 2001;38(5):1348-1354. doi:10.1016/S0735-1097(01)01563-7.
- Jong G-P, Ma T, Chou P, Shyu M-Y, Tseng W-K, Chang T-C. Reciprocal Changes in 12-Lead Electrocardiography Can Predict Left Main Coronary Artery Lesion in Patients With Acute Myocardial Infarction. Int Heart J. 2006;47(1):13-20. doi:10.1536/ihj.47.13.
- Sclarovsky S, Kjell N, Birnbaum Y. Manifestation of left main coronary artery stenosis is diffuse st depression in inferior and precordial leads on ECG. J Am Coll Cardiol. 2002;40(3):575-576. doi:10.1016/S0735-1097(02)02027-2.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):e78-e140. doi:10.1016/j.jacc.2012.11.019.
- Smith SW. Updates on the Electrocardiogram in Acute Coronary Syndromes. Curr Emerg Hosp Med Rep. 2013;1(1):43-52. doi:10.1007/s40138-012-0003-1.
- Pahlm US, Pahlm O, Wagner GS. The standard 11-lead ECG. J Electrocardiol. 1996;29:270-274. doi:10.1016/S0022-0736(96)80074-4.
- Gorgels APM, Engelen DJM, Wellens HJJ. Lead aVR, a mostly ignored but very valuable lead in clinical electrocardiography**Editorials published in the Journal of the American College of Cardiologyreflect the views of the authors and do not necessarily represent the views of JACCor the American College of Cardiology. J Am Coll Cardiol. 2001;38(5):1355-1356. doi:10.1016/S0735-1097(01)01564-9.
- Williamson K, Mattu A, Plautz CU, Binder A, Brady WJ. Electrocardiographic applications of lead aVR. Am J Emerg Med. 2006;24(7):864-874. doi:10.1016/j.ajem.2006.05.013.
- Kosuge M, Ebina T, Hibi K, et al. An Early and Simple Predictor of Severe Left Main and/or Three-Vessel Disease in Patients With Non–ST-Segment Elevation Acute Coronary Syndrome. Am J Cardiol. 2011;107(4):495-500. doi:10.1016/j.amjcard.2010.10.005.
- Barrabés JA, Figueras J, Moure C, Cortadellas J, Soler-Soler J. Prognostic Value of Lead aVR in Patients With a First Non–ST-Segment Elevation Acute Myocardial Infarction. Circulation. 2003;108(7):814-819. doi:10.1161/01.CIR.0000084553.92734.83.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, Laboratory, Roentgenographic, and Electrocardiographic Findings in Patients with Acute Pulmonary Embolism and No Pre-Existing Cardiac or Pulmonary Disease. Chest. 1991;100(3):598-603. doi:10.1378/chest.100.3.598.
- Marchick MR, Courtney DM, Kabrhel C, et al. 12-Lead ECG Findings of Pulmonary Hypertension Occur More Frequently in Emergency Department Patients With Pulmonary Embolism Than in Patients Without Pulmonary Embolism. Ann Emerg Med. 2010;55(4):331-335. doi:10.1016/j.annemergmed.2009.07.025.
- Becattini C, Vedovati MC, Agnelli G. Prognostic Value of Troponins in Acute Pulmonary Embolism: A Meta-Analysis. Circulation. 2007;116(4):427-433. doi:10.1161/CIRCULATIONAHA.106.680421.
- Gibson NS, Sohne M, Buller HR. Prognostic value of echocardiography and spiral computed tomography in patients with pulmonary embolism. Curr Opin Pulm Med. 2005;11(5):380-384.
- Douma RA, Hofstee HMA, Schaefer-Prokop C, et al. Comparison of 4- and 64-slice CT scanning in the diagnosis of pulmonary embolism. Thromb Haemost. 2010;103(1):242-246. doi:10.1160/TH09-06-0406.
- Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary Embolism Detection: Prospective Evaluation of Dual-Section Helical CT versus Selective Pulmonary Arteriography in 157 Patients. Radiology. 2000;217(2):447-455. doi:10.1148/radiology.217.2.r00nv01447.
- Carrier M, Righini M, Wells PS, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 2010;8(8):1716-1722. doi:10.1111/j.1538-7836.2010.03938.x.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease:CHEST Guideline and Expert Panel Report. CHEST. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026.
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): New Insights Into an Old Disease. JAMA. 2000;283(7):897-903. doi:10.1001/jama.283.7.897.
- Jagannath A, Sos T, Lockhart S, Saddekni S, Sniderman K. Aortic dissection: a statistical analysis of the usefulness of plain chest radiographic findings. Am J Roentgenol. 1986;147(6):1123-1126. doi:10.2214/ajr.147.6.1123.
- Kodolitsch Y von, Nienaber CA, Dieckmann C, et al. Chest radiography for the diagnosis of acute aortic syndrome. Am J Med. 2004;116(2):73-77. doi:10.1016/j.amjmed.2003.08.030.
- Kodolitsch Y von, Schwartz AG, Nienaber CA. Clinical Prediction of Acute Aortic Dissection. Arch Intern Med. 2000;160(19):2977-2982. doi:10.1001/archinte.160.19.2977.
- Kosuge M, Uchida K, Imoto K, et al. Frequency and Implication of ST-T Abnormalities on Hospital Admission Electrocardiograms in Patients With Type A Acute Aortic Dissection. Am J Cardiol. 2013;112(3):424-429. doi:10.1016/j.amjcard.2013.03.050.
- Vrsalovic M. Prognostic effect of cardiac troponin elevation in acute aortic dissection: A meta-analysis. Int J Cardiol. 2016;214(Supplement C):277-278. doi:10.1016/j.ijcard.2016.03.230.
- Black JH, Manning WJ. Clinical features and diagnosis of acute aortic dissection – UpToDate. https://www-uptodate-com.foyer.swmed.edu/contents/clinical-features-and-diagnosis-of-acute-aortic-dissection?source=machineLearning&search=aortic%20dissection&selectedTitle=1~150§ionRank=2&anchor=H17#H2152408195. Accessed October 16, 2017.
- Moore AG, Eagle KA, Bruckman D, et al. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD). Am J Cardiol. 2002;89(10):1235-1238. doi:10.1016/S0002-9149(02)02316-0.
- Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The Diagnosis of Thoracic Aortic Dissection by Noninvasive Imaging Procedures. N Engl J Med. 1993;328(1):1-9. doi:10.1056/NEJM199301073280101.
- Asha SE, Miers JW. A Systematic Review and Meta-analysis of D-dimer as a Rule-out Test for Suspected Acute Aortic Dissection. Ann Emerg Med. 2015;66(4):368-378. doi:10.1016/j.annemergmed.2015.02.013.
- Members WG, Hiratzka LF, Bakris GL, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13):e266-e369. doi:10.1161/CIR.0b013e3181d4739e.
- Tweet MS, Hayes SN, Pitta SRM, et al. Clinical Features, Management, and Prognosis of Spontaneous Coronary Artery Dissection. Circulation. 2012;126(5):579-588. doi:10.1161/CIRCULATIONAHA.112.105718.
- Mortensen K h., Thuesen L, Kristensen I b., Christiansen E h. Spontaneous coronary artery dissection: A Western Denmark Heart Registry Study. Catheter Cardiovasc Interv. 2009;74(5):710-717. doi:10.1002/ccd.22115.
- Alfonso F. Spontaneous Coronary Artery Dissection: New Insights From the Tip of the Iceberg? [Editorial]. Circulation. 2012;126(6):667-670. doi:10.1161/CIRCULATIONAHA.112.122093.
- Saw J, Aymong E, Sedlak T, et al. Spontaneous Coronary Artery Dissection: Association With Predisposing Arteriopathies and Precipitating Stressors and Cardiovascular Outcomes. Circ Cardiovasc Interv. 2014;7(5):645-655. doi:10.1161/CIRCINTERVENTIONS.114.001760.
- Saw J, Aymong E, Mancini GBJ, Sedlak T, Starovoytov A, Ricci D. Nonatherosclerotic Coronary Artery Disease in Young Women. Can J Cardiol. 2014;30(7):814-819. doi:10.1016/j.cjca.2014.01.011.
- Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523-1529. doi:10.1093/eurheartj/ehl032.
- Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408-417. doi:10.1016/j.ahj.2007.11.008.
- Nef HM, Möllmann H, Kostin S, et al. Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur Heart J. 2007;28(20):2456-2464. doi:10.1093/eurheartj/ehl570.
- Templin C, Ghadri JR, Diekmann J, et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373(10):929-938. doi:10.1056/NEJMoa1406761.
- Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52(4):274-288. doi:10.1016/j.pcad.2009.11.006.
- Hufnagel G, Pankuweit S, Richter A, Schönian U, Maisch B, Investigators for the E. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID) First Epidemiological Results. Herz. 2000;25(3):279-285. doi:10.1007/s000590050021.
- Karjalainen J, Heikkilä J. Incidence of three presentations of acute myocarditis in young men in military service. A 20-year experience. Eur Heart J. 1999;20(15):1120-1125. doi:10.1053/euhj.1998.1444.
- Angelini A, Calzolari V, Calabrese F, et al. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart. 2000;84(3):245-250. doi:10.1136/heart.84.3.245.
- Miklozek CL, Crumpacker CS, Royal HD, Come PC, Sullivan JL, Abelmann WH. Myocarditis presenting as acute myocardial infarction. Am Heart J. 1988;115(4):768-776. doi:10.1016/0002-8703(88)90877-0.
- Dec GW, Waldman H, Southern J, Fallon JT, Hutter AM, Palacios I. Viral myocarditis mimicking acute myocardial infarction. J Am Coll Cardiol. 1992;20(1):85-89. doi:10.1016/0735-1097(92)90141-9.
- Caforio ALP, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636-2648. doi:10.1093/eurheartj/eht210.
- Lauer B, Niederau C, Kühl U, et al. Cardiac Troponin T in Patients With Clinically Suspected Myocarditis. J Am Coll Cardiol. 1997;30(5):1354-1359. doi:10.1016/S0735-1097(97)00317-3.
- Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of Cardiac Troponin I Associated With Myocarditis: Experimental and Clinical Correlates. Circulation. 1997;95(1):163-168.
- Mason JW, O’Connell JB, Herskowitz A, et al. A Clinical Trial of Immunosuppressive Therapy for Myocarditis. N Engl J Med. 1995;333(5):269-275. doi:10.1056/NEJM199508033330501.
- Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med Boston. 2000;342(15):1077-1084.
- Dudzinski DM, Mak GS, Hung JW. Pericardial Diseases. Curr Probl Cardiol. 2012;37(3):75-118. doi:10.1016/j.cpcardiol.2011.10.002.
- Seferović PM, Ristić AD, Maksimović R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18(3):255-266. doi:10.1007/s10741-012-9335-x.
- Buiatti A, Merlo M, Pinamonti B, De Biasio M, Bussani R, Sinagra G. Clinical presentation and long-term follow-up of perimyocarditis. J Cardiovasc Med. 2013;14(3):235-241. doi:10.2459/JCM.0b013e328351da6e.
- Imazio M, Brucato A, Maestroni S, et al. Prevalence of C-Reactive Protein Elevation and Time Course of Normalization in Acute PericarditisClinical Perspective: Implications for the Diagnosis, Therapy, and Prognosis of Pericarditis. Circulation. 2011;123(10):1092-1097. doi:10.1161/CIRCULATIONAHA.110.986372.
- LeWinter MM. Acute Pericarditis. N Engl J Med. 2014;371(25):2410-2416. doi:10.1056/NEJMcp1404070.
- Maron BJ. Hypertrophic Cardiomyopathy: A Systematic Review. JAMA. 2002;287(10):1308-1320. doi:10.1001/jama.287.10.1308.
- Semsarian C, Ingles J, Maron MS, Maron BJ. New Perspectives on the Prevalence of Hypertrophic Cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019.
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy. The Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6.
- Kelly BS, Mattu A, Brady WJ. Hypertrophic cardiomyopathy: electrocardiographic manifestations and other important considerations for the emergency physician. Am J Emerg Med. 2007;25(1):72-79. doi:10.1016/j.ajem.2006.04.017.
- Nishimura RA, Holmes DRJ. Hypertrophic Obstructive Cardiomyopathy. N Engl J Med. 2004;350(13):1320-1327. doi:10.1056/NEJMcp030779.
- Kawasaki T, Azuma A, Kuribayashi T, et al. Resting ST-segment depression predicts exercise-induced subendocardial ischemia in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2006;107(2):267-274. doi:10.1016/j.ijcard.2005.03.031.
- Zhang C, Liu R, Yuan J, et al. Significance and Determinants of Cardiac Troponin I in Patients With Obstructive Hypertrophic Cardiomyopathy. Am J Cardiol. 2015;116(11):1744-1751. doi:10.1016/j.amjcard.2015.09.006.
- McLeod CJ, Ackerman MJ, Nishimura RA, Tajik AJ, Gersh BJ, Ommen SR. Outcome of Patients With Hypertrophic Cardiomyopathy and a Normal Electrocardiogram. J Am Coll Cardiol. 2009;54(3):229-233. doi:10.1016/j.jacc.2009.02.071.
- Shavelle DM, Otto CM. Aortic Stenosis. In: Cardiology. 3rd ed. Philadelphia, PA: Elsevier; 2010:1275-1284. https://www-clinicalkey-com.foyer.swmed.edu/#!/content/book/3-s2.0-B9780723434856000936?scrollTo=%23hl0000312.
- Julius BK, Spillmann M, Vassalli G, Villari B, Eberli FR, Hess OM. Angina Pectoris in Patients With Aortic Stenosis and Normal Coronary Arteries: Mechanisms and Pathophysiological Concepts. Circulation. 1997;95(4):892-898. doi:10.1161/01.CIR.95.4.892.
- Otto CM, Burwash IG, Legget ME, et al. Prospective Study of Asymptomatic Valvular Aortic Stenosis: Clinical, Echocardiographic, and Exercise Predictors of Outcome. Circulation. 1997;95(9):2262-2270. doi:10.1161/01.CIR.95.9.2262.
- Carabello BA. Valvular Heart Disease. In: Goldman-Cecil Medicine. 25th ed. Philadelphia, PA: Elsevier/Saunders; 2016:461-473.e2. https://www-clinicalkey-com.foyer.swmed.edu/#!/content/book/3-s2.0-B9781455750177000751?scrollTo=%23hl0001055.
- Chen J, Okin PM, Roman MJ, et al. Combined rest and exercise electrocardiographic repolarization findings in relation to structural and functional abnormalities in asymptomatic aortic regurgitation. Am Heart J. 1996;132(2, Part 1):343-347. doi:10.1016/S0002-8703(96)90431-7.
- Kligfield P, Ameisen O, Okin PM, et al. Relationship of the electrocardiographic response to exercise to geometric and functional findings in aortic regurgitation. Am Heart J. 1987;113(5):1097-1102. doi:10.1016/0002-8703(87)90918-5.
- Peacock WFI, De Marco T, Fonarow GC, et al. Cardiac Troponin and Outcome in Acute Heart Failure. N Engl J Med. 2008;358(20):2117-2126. doi:10.1056/NEJMoa0706824.
- Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O’Connor CM, Felker GM. Troponin Elevation in Heart Failure: Prevalence, Mechanisms, and Clinical Implications. J Am Coll Cardiol. 2010;56(14):1071-1078. doi:10.1016/j.jacc.2010.06.016.
- Parikh P, Jeremias A. Elevated Cardiac Troponin in the Absence of Acute Coronary Syndromes: Mechanism, Significance, and Prognosis. In: Cardiac Intensive Care. 2nd ed. Philadelphia, PA: Elsevier/Saunders; 2010:196-202. https://www-clinicalkey-com.foyer.swmed.edu/#!/content/book/3-s2.0-B9781416037736100151?scrollTo=%23hl0000283.
- Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid Measurement of B-Type Natriuretic Peptide in the Emergency Diagnosis of Heart Failure. N Engl J Med. 2002;347(3):161-167. doi:10.1056/NEJMoa020233.
- Januzzi JL, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP Investigation of Dyspnea in the Emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948-954. doi:10.1016/j.amjcard.2004.12.032.
- Liteplo AS, Marill KA, Villen T, et al. Emergency Thoracic Ultrasound in the Differentiation of the Etiology of Shortness of Breath (ETUDES): Sonographic B-lines and N-terminal Pro-brain-type Natriuretic Peptide in Diagnosing Congestive Heart Failure. Acad Emerg Med. 2009;16(3):201-210. doi:10.1111/j.1553-2712.2008.00347.x.
- Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-591. doi:10.1007/s00134-012-2513-4.
- Torbicki A, Kurzyna M, Kuca P, et al. Detectable Serum Cardiac Troponin T as a Marker of Poor Prognosis Among Patients With Chronic Precapillary Pulmonary Hypertension. Circulation. 2003;108(7):844-848. doi:10.1161/01.CIR.0000084544.54513.E2.
- Roy AK, McCullagh BN, Segurado R, et al. Detection of High-Sensitivity Troponin in Outpatients With Stable Pulmonary Hypertension Identifies a Subgroup at Higher Risk of Adverse Outcomes. J Card Fail. 2014;20(1):31-37. doi:10.1016/j.cardfail.2013.12.001.
- Gravning J, Askevold ET, Nymo SH, et al. Prognostic Effect of High-Sensitive Troponin T Assessment in Elderly Patients With Chronic Heart FailureClinical Perspective: Results From the CORONA Trial. Circ Heart Fail. 2014;7(1):96-103. doi:10.1161/CIRCHEARTFAILURE.113.000450.
- La Vecchia L, Mezzena G, Zanolla L, et al. Cardiac troponin I as diagnostic and prognostic marker in severe heart failure. J Heart Lung Transplant. 2000;19(7):644-652. doi:10.1016/S1053-2498(00)00120-0.
- Pavasini R, d’Ascenzo F, Campo G, et al. Cardiac troponin elevation predicts all-cause mortality in patients with acute exacerbation of chronic obstructive pulmonary disease: Systematic review and meta-analysis. Int J Cardiol. 2015;191(Supplement C):187-193. doi:10.1016/j.ijcard.2015.05.006.
- Baillard C, Boussarsar M, Fosse J-P, et al. Cardiac troponin I in patients with severe exacerbation of chronic obstructive pulmonary disease. Intensive Care Med. 2003;29(4):584-589. doi:10.1007/s00134-003-1635-0.
- Stone IS, Petersen SE, Barnes NC. Raised troponin in COPD: clinical implications and possible mechanisms. Heart. 2013;99(2):71-72. doi:10.1136/heartjnl-2012-302969.
- Brekke PH, Omland T, Holmedal SH, Smith P, Søyseth V. Troponin T elevation and long-term mortality after chronic obstructive pulmonary disease exacerbation. Eur Respir J. 2008;31(3):563-570. doi:10.1183/09031936.00015807.






